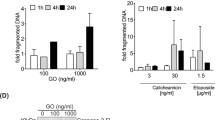
Abstract
Resistance of leukemic cells to chemotherapy frequently occurs in patients with acute leukemia, which may be caused by alterations in common apoptotic pathways. Controversy exists whether cytostatic agents induce the mitochondrial or death receptor pathway of apoptosis. In the mitochondrial pathway cytochrome C release and caspase-9 activation play a central role in the induction of apoptosis, while formation of a Death Inducing Signaling Complex (DISC) and caspase-8 activation have been reported to be essential in death receptor-induced apoptosis. Here, we show in human derived myeloid and lymphoblastic leukemia cell lines that caspase-8 plays a more important role than previously expected in apoptosis mediated via the mitochondrial pathway. We demonstrated in these malignant cells chemotherapy-induced apoptosis independent of the death receptor pathway, since blocking this pathway using a retroviral construct encoding Flice inhibitory protein (FLIP) did not inhibit drug-induced apoptosis or caspase-8 activation, while overexpression of Bcl-2 completely inhibited both events. Furthermore, we showed that activation of caspase-8 by cytostatic agents occurred downstream from mitochondria. Since caspase-8 plays a central role in both death receptor- and chemotherapy-induced apoptosis of malignant cells from patients with acute leukemia, therapeutic strategies focusing at modulation and activation of caspase-8 may be successful in the treatment of drug-resistant malignancies.
Similar content being viewed by others
Reference
Zittoun RA, Mandelli F, Willemze R et al (1995) Autologous or allogeneic bone-marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. N Engl J Med 332:217–23
Lowenberg B, Downing JR, Burnett A (1999) Medical progress - acute myeloid leukemia. N Engl J Med 341:1051–062
Pui CH, Evans WE (1998) Acute lymphoblastic leukemia. N Engl J Med 339:605–15
Chauncey TR (2001) Drug resistance mechanisms in acute leukemia. Curr Opin Oncol 13:21–6
List AF (1996) Role of multidrug resistance and its pharmacological modulation in acute myeloid leukemia. Leukemia 10:937–42
Maung ZT, Maclean FR, Reid MM et al (1994) The relationship between Bcl-2 expression and response to chemotherapy in acute-leukemia. Br J Haematol 88:105–09
Pirker R, Wallner J, Geissler K et al (1991) Mdr1 gene-expression and treatment outcome in acute myeloid-leukemia. J Natl Cancer Inst 83:708–12
Stoetzer OJ, Nussler V, Darsow M et al (1996) Association of bcl-2, bax, bcl-xL and inteuleukin-1 beta-converting enzyme expression with initial response to chemotherapy in acute myeloid leukemia. Leukemia 10:S18–S22
Schimmer AD, Pedersen IM, Kitada S et al (2003) Functional blocks in caspase activation pathways are common in leukemia and predict patient response to induction chemotherapy. Cancer Res 63:1242–248
Thornberry NA, Lazebnik Y (1998) Caspases: Enemies within. Science 281:1312–316
Li P, Nijhawan D, Budihardjo I et al (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–89
Friesen C, Herr I, Krammer PH, Debatin KM (1996) Involvement of the CD95 (APO-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med 2:574–77
Friesen C, Fulda S, Debatin KM (1997) Deficient activation of the CD95 (APO-1/Fas) system in drug-resistant cells. Leukemia 11:1833–841
Fulda S, Sieverts H, Friesen C, Herr I, Debatin KM (1997) The CD95 (APO-1/Fas) system mediates drug-induced apoptosis in neuroblastoma cells. Cancer Res 57:3823–829
Herr I, Wilhelm D, Bohler T, Angel P, Debatin KM (1997) Activation of CD95 (APO-1/Fas) signaling by ceramide mediates cancer therapy-induced apoptosis. Embo J 16:6200–208
Muller M, Strand S, Hug H et al (1997) Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J Clin Invest 99:403–13
Chen GQ, Goeddel DV (2002) TNF-R1 signaling: A beautiful pathway. Science 296:1634–635
Fulda S, Meyer E, Friesen C, Susin SA, Kroemer G, Debatin KM (2001) Cell type specific involvement of death receptor and mitochondrial pathways in drug-induced apoptosis. Oncogene 20:1063–075
Nagata S (1999) Fas ligand-induced apoptosis. Annu Rev Genet 33:29–5
Tourneur U, Delluc S, Levy V et al (2004) Absence or low expression of Fas-associated protein with death domain in acute myeloid leukemia cells predicts resistance to chemotherapy and poor outcome. Cancer Res 64:8101–108
Kitada S, Pedersen IM, Schimmer AD, Reed JC (2002) Dysregulation of apoptosis genes in hematopoietic malignancies. Oncogene 21:3459–474
Schimmer AD, Hedley DW, Penn LZ, Minden MD (2001) Receptor- and mitochondrial-mediated apoptosis in acute leukemia: A translational view. Blood 98:3541–553
Chadderton A, Villeneuve DJ, Gluck S et al (2000) Role of specific apoptotic pathways in the restoration of paclitaxel-induced apoptosis by valspodar in doxorubicin-resistant MCF-7 breast cancer cells. Breast Cancer Res Treat 59:231–44
Grotzer MA, Eggert A, Zuzak TJ et al (2000) Resistance to TRAIL-induced apoptosis in primitive neuroectodermal brain tumor cells correlates with a loss of caspase-8 expression. Oncogene 19:4604–610
Teitz T, Wei T, Valentine MB et al (2000) Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med 6:529–35
Wesselborg S, Engels IH, Rossmann E, Los M, Schulze-Osthoff K (1999) Anticancer drugs induce caspase-8/FLICE activation and apoptosis in the absence of CD95 receptor/ligand interaction. Blood 93:3053–063
Irmler M, Thome M, Hahne M et al (1997) Inhibition of death receptor signals by cellular FLIP. Nature 388:190–95
Thome M, Schneider P, Hofmann K et al (1997) Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517–21
Kluck RM, BossyWetzel E, Green DR, Newmeyer DD (1997) The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 275:1132–136
Yang J, Liu XS, Bhalla K et al (1997) Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 275:1129–132
Lange B, Valtieri M, Santoli D et al (1987) Growth-Factor Requirements of Childhood Acute-Leukemia—Establishment of Gm-Csf Dependent Cell-Lines. Blood 70:192–99
Jedema I, Barge RM, Willemze R, Falkenburg JH (2003) High susceptibility of human leukemic cells to Fas-induced apoptosis is restricted to G1 phase of the cell cycle and can be increased by interferon treatment. Leukemia 17:576–84
Jedema I, Van Der Werff NM, Barge RM, Willemze R, Falkenburg JH (2004) New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood 103:2677–682
Veuger MJT, Honders MW, Willemze R, Barge RMY (2002) Deoxycytidine kinase expression and activity in patients with resistant versus sensitive acute myeloid leukemia. Eur J Haematol 69:171–78
Kinsella TM, Nolan GP (1996) Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther 7:1405–413
Heemskerk MHM, de Paus RA, Lurvink EGA et al (2001) Dual HLA class I and class II restricted recognition of alloreactive T lymphocytes mediated by a single T cell receptor complex. Proc Natl Acad Sci USA 98:6806–811
Hanenberg H, Xiao XL, Dilloo D, Hashino K, Kato I, Williams DA (1996) Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat Med 2:876–82
Rehemtulla A, Hamilton CA, Chinnaiyan AM, Dixit VM (1997) Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1). J Biol Chem 272:25783–5786
Micheau O, Solary E, Hammann A, Dimanche-Boitrel MT (1999) Fas ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs. J Biol Chem 274:7987–992
Chinnaiyan AM, ORourke K, Lane BR, Dixit VM (1997) Interaction of CED-4 with CED-3 and CED-9: A molecular framework for cell death. Science 275:1122–126
Kim PKM, Mahidhara R, Seol DW (2001) The role of caspase-8 in resistance to cancer chemotherapy. Drug Resist Updates 4:293–96
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a grant of the Dutch Cancer Society/KWF Kankerbestrijding: 99-2122.
Rights and permissions
About this article
Cite this article
de Vries, J.F., Wammes, L.J., Jedema, I. et al. Involvement of caspase-8 in chemotherapy-induced apoptosis of patient derived leukemia cell lines independent of the death receptor pathway and downstream from mitochondria. Apoptosis 12, 181–193 (2007). https://doi.org/10.1007/s10495-006-0526-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-0526-6