Abstract
Ticks attaching to ear canals of humans and animals are the cause of otoacariasis, common in rural areas of Nepal. The plant Clerodendrum viscosum is used in multiple indigenous systems of medicine by ethnic communities in the Indo-Nepali-Malaysian region. Visiting the Chitwan National Park, we learned that in indigenous medicine, flower extract of C. viscosum is utilized to treat digestive disorders and extracts from leaves as tick repellent to prevent ticks from invading or to remove them from the ear canal. The objective of our study was to provide support to indigenous medicine by characterizing the in vivo effect of leave extracts on ticks under laboratory conditions and its phytochemical composition. We collected plant parts of C. viscosum (leaves and flowers) and mango (Mangifera indica) leaves at the Chitwan National Park, previously associated with repellent activity to characterize their effect on Ixodes ricinus ticks by in vivo bioassays. A Q-ToF high-resolution analysis (HPLC-ESI-QToF) was conducted to elucidate phenolic compounds with potential repellent activity. Clerodendrum viscosum and M. indica leaf extracts had the highest tick repellent efficacy (%E = 80–100%) with significant differences when compared to C. viscosum flowers extracts (%E = 20–60%) and phosphate-buffered saline. Phytochemicals with tick repellent function as caffeic acid, fumaric acid and p-coumaric acid glucoside were identified in C. viscosum leaf extracts by HPLC-ESI-QToF, but not in non-repellent flower extracts. These results support the Nepali indigenous medicine application of C. viscosum leaf extracts to repel ticks. Additional research is needed for the development of natural and green repellent formulations to reduce the risks associated with ticks resistant to acaricides.
Similar content being viewed by others
Introduction
Ticks of genera such as Amblyomma, Dermacentor, Haemaphysalis, Hyalomma, Ixodes, and Rhipicephalus are widespread throughout Nepal including the Chitwan National Park with associated health risks for humans and wildlife (Pun et al. 2018). Intra-aural ticks attached to the external auditory canal may cause peripheral facial nerve paralysis (Doğan et al. 2012; Kularatne et al. 2018). Ticks and (other) mites attached within the ear canal are the cause of otoacariasis common in rural areas of Nepal and other countries in both humans and animals, making ectoparasite removal hard with partially efficient treatment interventions (Fegan and Glennon 1996; Indudharan et al. 1999; Somayaji and Rajeshwari 2007; Doğan et al. 2012; Cakabay et al. 2016; Kwak et al. 2021). Due to culture and economic resources, rural Asiatic areas have developed traditional methods for control of local parasites or food preservation (Salehi et al. 2018; Riaz et al. 2020). Indeed, research on these methods may provide new alternatives for ‘green’ chemical formulations. It has been recently reported that some compounds synthetically derived from botanical sources have acaricidal activity against Ixodes scapularis similar to repellents in the market, supporting a scientific base to traditional and natural medicine and arising new perspective for green chemical-based strategies (Lee et al. 2022).
The plant Clerodendrum viscosum Vent (Lamiaceae; synonym Clerodendrum infortunatum L.; hill glory bower or Bhatti in Nepali) germinates in June-July and flowers in February-March, and is distributed throughout tropical and subtropical regions of Asia, Africa and the Pacific with particular relevance in the Indo-Nepali-Malaysian region (Srivastava et al. 2021). Together with other natural resources, C. viscosum has been described as a traditional botanical medicine and is the primary mode of health care by local medical experts in indigenous systems of Nepal and Pakistan communities (Bhattarai et al. 2010; Ishtiaq et al. 2021). In these rural areas, C. viscosum is used as an effective tool for human health against cough/cold, itching, indigestion and abdominal pain (Bhattacharjee and Ray 2010). In the Unani medical system, leaf decoction has been applicated in rheumatism system and seed power as vermicide treatment (Singh et al. 1997). Clerodendrum viscosum plant extracts and their phytoconstituents have shown anti-inflammatory, antioxidant, antidiabetic, anticancer, immunomodulatory, hemagglutination, antimicrobial, insecticidal and hepatoprotection pharmacological properties under laboratory conditions (Bird 1961; Luitel et al. 2014; Nandi and Lyndem 2016; Ishtiaq et al. 2021; Shendge et al. 2021a, b; Srivastava et al. 2021) and used in asthma, malaria or blood and respiratory system diseases (Nandi and Lyndem 2016; Joshi et al. 2020). A gas chromatography-mass spectrometry (GC-MS) analysis on methanol extract of C. viscosum demonstrated the presence of steroids, triterpenoids and flavonoids (Ghosh et al. 2015). The two major bioactive compounds found by Ghosh et al. (2015) are N,N-dimethylglycine and 3-deoxy-d-mannoic lactone previously reported to have immune modulating properties and antibacterial activity (Graber et al. 1981). In vitro studies have shown apoptotic activity of bioflavonoid apigenin isolated from Clerodendrum leaves (Shendge et al. 2017, 2021a) and anticancer activity of 70% methanolic extracts (Shendge et al. 2021a). However, there is no information for its potential as tick repellent.
Natural repellents are an approach to decrease and overcome the risks associated with tick acaricide resistance and for integrated control of ticks and tick-borne diseases (de la Fuente 2018; Quadros et al. 2020; Wang et al. 2022; Sharma et al. 2022). The main phytochemicals identified in C. viscosum are monoterpenoids, diterpenoids, triterpenoids, glycosides, saponins, steroids, and flavonoids (Nandi and Lyndem 2016; Quadros et al. 2020; Srivastava et al. 2021). Plant-derived alkaloids, monoterpenoids such as thymol, carvacrol and linalool and essential oils diterpenoids have been characterized as potential acaricide with repellent and toxic activity against ticks (Gross et al. 2017; Schubert et al. 2017; Tabari et al. 2017; Adenubi et al. 2018a; Soutar et al. 2019; Nwanade et al. 2020; Quadros et al. 2020; Luns et al. 2021).
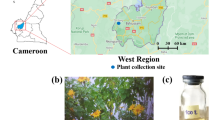
When we visited the Chitwan National Park we learned that in Nepali indigenous medicine, extracts of C. viscosum plants were used for treatment of digestive disorders (flowers), hemagglutination (fruits) and tick repellent (leaves). Extracts from other Clerodendrum species (Clerodendrum glabrum, also known as Rotheca glabrum) were used as repellent against adults of Rhipicephalus appendiculatus and for the control of cattle ticks in South Africa (Mawela et al. 2019). Clerodendrum plants are also used in this area for feeding goats. Although insects can be found on C. viscosum plants, community members claim that ticks are rarely found in areas with these plants with protection to humans and wildlife (Fig. 1A). To prevent ticks from invading or to remove them from the ear canal, Nepalis construct a funnel with leaves, place it on the ear canal and add a burning coal piece to extract plant leaf juice with repellent activity (Fig. 1A). Based on this information, we collected at the Chitwan National Park Bhatti C. viscosum leaves and flowers together with mango (Mangifera indica) leaves also associated with repellent activity (Alwala et al. 2010) to characterize their in vivo effect on ticks under laboratory conditions and phytochemical composition.
Materials and methods
Plant extracts
Extracts from leaves and flowers of C. viscosum (Fig. 1A) and leaves of mango were prepared. All extracts were used to evaluate tick repellency. The plant name is recorded by The World Flora Online List (previous www.theplantlist.org), record kew-43,162. Samples were collected in February 2022 at the Nepali Chitwan National Park. To mimic conditions used by indigenous medicine to remove/repel ticks, plant leaves and flowers were separated manually, and the aqueous plant extract prepared shortly before application. Leaves or flowers were ground in 2-ml tubes with 3-mm-diameter G25 chrome steel (AISI 52,100) beads (Amazon, Ciudad Real, Spain) using a vortex mixer (Stuart Scientific, Merck, Germany) as previously described (Huynh et al. 2017). Grinded plant components were then smashed using a mortar and pestle in 5% wt/wt sterile distilled water for 10 min and then stored at room temperature overnight. The aqueous extracts were filtered using a 0.2 mm filter (Millipore, Burlington, MA, USA) to remove particulate matter. Dimethyl sulfoxide (DMSO) extracts were prepared by cutting plant samples in small pieces and smashing them with a mortar and pestle. Aliquots of 3 ml of DMSO were added during the smashing process and plant extracts were placed in agitation set at 4 °C overnight with agitation in an SB3 Stuart rotating shaker (Biolab, Barcelona, Spain) and then centrifuged at 4000× g for 10 min to collect supernatant. Negative controls were based on phosphate-buffered saline (PBS) for both aqueous and DMSO plant extracts.
Ixodes ricinus ticks
Experimental I. ricinus ticks were obtained from a laboratory colony maintained at the Institute of Parasitology, Biology Centre of the Czech Academy of Sciences (BC CAS), Ceske Budejovice, Czech Republic (Hartmann et al. 2018). Same age unfed female and male ticks were provided by D. Sojka and L. Grubhoffer (CAS). Ticks were maintained in the rearing facility at CAS under controlled conditions (L12:D12 photoperiod, 24 °C and 95% r.h.). All laboratory animals were treated in accordance with the Animal Protection Law of the Czech Republic No. 246/1992 Sb., ethics approval No. 25/2018. The study was approved by the Institute of Parasitology, Biology Centre CAS and Central Committee for Animal Welfare, Czech Republic (Protocol No. 1/2015).
Petri dish and tick climbing repellency bioassays
To evaluate tick repellent activity, plant aqueous and DMSO extracts were assayed using previously validated Petri dish and tick climbing repellency bioassays (Fig. 1B) (Dautel 2004; Gliniewicz et al. 2017; Adenubi et al. 2018b).
Petri dish repellency bioassay
A filter paper with an open hole (approx. 35 mm diameter) in the center was placed on the Petri dish. Petri dishes were maintained in a chamber without contact with observers at 25 °C. Plant extracts or PBS (0.5 ml) were applied to different sides of the paper and after 5 min to dry, 10 unfed I. ricinus ticks (1:1 female:male) under questing behavior were added in the dish open center. Ticks can either enter and remain on the surface treated with plant extract or on the control PBS-treated surface. Tick counts were recorded after 10 min. Experiments with 10 ticks each were repeated 2× for each treatment. Repellent efficacy (%E) was calculated as [(total number of ticks – number of ticks on plant extract filter side)/10] × 100%. Similar results were obtained with plant aqueous and DMSO extracts and thus used together to evaluate significant repellency by comparison between both sides of the dish and groups, using a one-way ANOVA test followed by post-hoc Tukey honestly significant difference (HSD) test to separate means, applying the Bonferroni–Holm method (α = 0.05, n = 4 biological replicates).
Tick climbing repellency bioassay
To provide additional support, a single trial was conducted under tick climbing repellency bioassay conditions. Plant aqueous extracts or PBS (0.4 ml) were added to filter paper strips and placed inside surface of Corning 15-ml centrifuge polypropylene, conical bottom tubes (Merck, Rahway, NJ, USA). After 5 min to dry, 10 unfed I. ricinus ticks (1:1 female:male) under questing behavior were added to the bottom of the tube. The ticks climbing on the filter papers and thus not repelled by treatment were counted 10 min after and the percentages of ticks climbing on were calculated and compared between groups.
Tick behavior
The behavior of selected ticks was recorded in the border of the filter paper between plant extracts and PBS control (Supplementary Video_1 and Supplementary Video_2).
Q-ToF high-resolution mass spectrometry analysis (HPLC-ESI-QToF)
The identification of phenolic compounds presented in both aqueous C. viscosum plant extracts (flower and leaves) was carried out by HPLC Agilent 1260 system coupled to a 6545 quadrupole-time of flight (Q-ToF) mass spectrometer detector (Agilent, Waldbronn, Germany). The Q-ToF used a Dual Jet Stream Electrospray Ionization (Dual AJS-ESI) source operated in both positive and negative ionization modes following the methods previously described by Torres-Vega et al. (2020) and Bordiga et al. (2013). For the positive ionization mode, the following parameters were set: capillary voltage, 3500 V; fragmentor, 150; nozzle voltage, 1000 V; gas temperature, 350 °C; gas flow 8 l/min; nebulizer, 40 psig; sheath gas temperature, 400 °C; sheath gas flow, 10 l/min; acquisition range, 100–1200 m/z. Samples were analysed after injection of 10 µl of each extract on a Zorbax Eclipse Plus C18 Rapid Resolution HD column (2.1 × 50 mm, 1.8 μm particle size; Agilent, Santa Clara, CA, USA), thermostat at 40 °C and a flow rate of 0.3 ml/min. The solvent system was 0.1% formic acid for solvent A and 0.1% formic acid in methanol for solvent B. The elution gradient was (time, % of solvent B): 0 min, 7%; 10 min, 20%; 40 min, 75%; 46.5 min, 95%; and 56 min, 7%. For the negative ionization mode, the parameters were set: capillary voltage, 3500 V; fragmentor, 150 V; nozzle voltage, 300 V; gas temperature, 300 °C; gas flow, 11 l/min; nebulizer, 20 psig; sheath gas temperature, 350 °C; sheath gas flow 11 l/min and acquisition range, 100–1200 m/z. Samples (10 µl) were analysed into an Acentis C18 reversed phase column (150 × 4.6 mm, 2.7 μm particle size; Supelco, Darmstadt, Germany), thermostat at 16 °C with a flow rate of 0.3 ml/min. The composition of mobile phase was the same with the positive ionization mode with the employed gradient (time, % of solvent B): 0 min, 7%; 25 min, 32%; 40 min, 57%; 50 min, 67%; 55 min, 97%; 65 min, 97%; and 70 min, 7%. The control software was Mass Hunter Workstation v.B.06.11 (Agilent, Santa Clara). Compounds were identified using the algorithm ‘Find by Formula’ that evaluated the mass accuracy together with the isotopic relative abundance and isotopic separation.
Results and discussion
Based on Nepali Chitwan National Park indigenous medicine practice, extracts from C. viscosum leaves and flowers and mango leaves were bioassayed for repellency against I. ricinus ticks using Petri dish and tick climbing bioassay techniques (Fig. 1B). Our results showed that C. viscosum and M. indica leaf extracts repelled ticks with highest tick repellent efficacy (%E) of 80–100% (Table 1) with significant differences compared with extract from flowers of C. viscosum (%E = 20–60%, Table 1) and PBS (Fig. 2A).
In agreement with the indigenous medicine application of C. viscosum flowers for the treatment of digestive disorders but not against ticks, the tick repellency effect was not significantly different from PBS control (Fig. 2A). Similar results were obtained in the single tick climbing repellency bioassay done to provide additional support to the Petri dish repellency bioassay (Fig. 2B).
Tick behavior was recorded for selected ticks in the border of the filter paper between plant extracts and PBS control. The procedure employed and results with C. viscosum leaves aqueous extract and M. indica leaves DMSO extract showed how some ticks moved immediately away from the plant extract while others explored the possibility of crossing the border but ended moving into the PBS control side (Supplementary Videos 1 and 2.).
These results provided evidence supporting the Nepali indigenous medicine application of extracts from C. viscosum leaves to repel ticks from invading or to remove them from the ear canal (Fig. 1B). Based on these findings, additional research should focus on identifying the C. viscosum phytochemicals with tick repellent function to further explore the development of natural formulations and reduce the risks associated with ticks resistant to acaricides. To address this objective, HPLC-ESI-QToF analysis was carried out and revealed the presence of phenolic compounds in flower, leaves or both aqueous extracts from C. viscosum (Table 2).
In the C. viscosum flower extract we found common flavonoids or derivatives also present in other plants, including other Clerodendrum species. Apigenin, apigenin glucuronide and acacetin glucuronide are chemical constituents also obtained from C. infortunatum (= C. viscosum) (Uddin et al. 2020; Srivastava et al. 2021) (Table 2). Apigenin is a common dietary flavonoid with anti-inflammatory, antioxidant or anti-bacterial properties (Yan et al. 2017). Indeed, it has been recently reported for being efficacious for the control of hematophagous mosquitoes Culex quinquefasciatus, causing significant damage in the midgut (Samuel et al. 2023). Acacetin is a flavone that has been mostly studied for its benefits on cardiovascular pathologies (Han et al. 2020; Liu et al. 2021; Wu et al. 2021).
Some molecules were found in both flowers and leaves of C. viscosum extracts via HPLC-ESI-QToF analysis (Table 2). Quinic acid is an abundant compound in different plant sources and present in coffee, cranberries or kiwifruit (Coppola et al. 1978; Heatherbell et al. 1980; Olthof et al. 2001) with clinical applications due to its capacity to modulate in vivo pancreatic beta-cell function and insulin secretion in mice (Heikkilä et al. 2019). Moreover, quinic acid protects plants from damage caused by invasive western flower thrips, Frankliniella occidentalis, becoming this cyclic polyol a potential application as a biocontrol agent in order to manage thrips (Liu et al. 2022).
The organic acids, glucaric acid and gluconic acid, found in both samples have been more studied in chemical industrial applications and for microbial interaction and growth, respectively (Nieto-Peñalver et al. 2014; Zhang et al. 2020). Interestingly, gluconic acid is produced by insect gut bacteria (Khan et al. 2020) and thus as shown in tick microbiota, could be targeted to reduce organism fitness (Mateos-Hernández et al. 2020).
The phenolic compound arbutin was found in both samples. This product is a derivative from the phenolic compound hydroquinone that has been widely found in leaves and used for the development of green products to control parasite infection in plants such as nematodes in tomato plants (Oliveira et al. 2019). This repellent capacity is supported by findings of natural isolates of Enterobacteriaceae that actively hydrolyse plant-derived compounds such as arbutin, to scape predation by bacterivorous amoeba and nematodes (Sonowal et al. 2013), and Lactobacillus present in tick and insect microbiota, also characterized as probiotic interventions produce acid from arbutin (Li and Gu 2022; Zhang et al. 2022; Gupta et al. 2023).
Relevant for the objective of this study, the compounds caffeic acid, fumaric acid and p-coumaric acid glucoside were found only in C. viscosum leaf extract with repellent activity shown in our assay against I. ricinus ticks (Fig. 2; Table 2). It has been reported that some flavonoids and phenolic active compounds are natural biomolecules that exhibit tyrosinase inhibitory activity, being this function essential for some insect and parasite metamorphosis (Panzuto et al. 2002; Kubo et al. 2003; Pantoja Pulido et al. 2017). Interestingly, caffeic acid and derivatives, isolated from natural resources such as leaves, seem to be the most studied compounds in terms of tick-borne diseases due to their antiviral effects, strong antioxidant activity and insect growth control (Pantoja Pulido et al. 2017). Caffeic acid and its derivatives have been found in plants of the genus Pulicaria, used in traditional medicine as insect deterrent (Malarz et al. 2023). This polyphenol has been deeply studied for its inhibitory effect on replication of hepatitis B and C virus (Wang et al. 2009; Shen et al. 2013). Apart for being responsible for aroma or colour, in East Asia it has been examined for its robust inhibitory effect against severe fever with thrombocytopenia syndrome (SFTS) (Ogawa et al. 2018), an emerging tick-borne pathology transmitted to humans due to several bites of tick species such as Haemaphysalis longicornis and Amblyomma testudinarium (Yun et al. 2014).
In case of fumaric acid, no data are available related to a specific protective tick-infection effect. Nevertheless, it has been elucidated for this organic acid to have powerful antimicrobial effect properties against several foodborne pathogens (Ramos et al. 2013; Salaheen et al. 2014; Park et al. 2016). Moreover, biosynthesis of functional metabolites such as fumaric acid is associated with survival and development at extreme high and low temperatures in Diptera such as Sitodiplosis mosellana (Huang et al. 2022), a process that may be also present during tick developmental and pathogen transmission dynamics (Gray et al. 2016).
Clerodendrum viscosum has been reported for its antimicrobial activity on bacteria and fungi (Rajakaruna et al. 2002; Oly et al. 2011; Amin et al. 2012), a capacity that might be associated to this type of natural compounds found on leaves. Antioxidant, anti-parasite and anti-mutagenesis activities have been also associated to p-coumarics phenolic acids due to the tyrosinase inhibitor capacity, also found in leave extract conjugated as p-coumaric acid glucoside (Pei et al. 2016; Oliveira et al. 2019; Lopes et al. 2020).
Other studies have reported the acaricidal activity against Rhipicephalus (Boophilus) sp. of derived-phytochemicals from traditional African plants such as terpenes, flavonoids and phenolic compounds, similar to those elucidated in this study (Alain et al. 2022). These secondary metabolites might also be involved in this function against other ticks. A previous GC-MS analysis of C. viscosum methanol extract also revealed the presence of compounds with antioxidant and/or antimicrobial activity (Ghosh et al. 2015). The identification in this study of new compounds present in a tick-repellent extract of leaves contribute to fully characterize C. viscosum plants together with those already found by other methodologies that may act in combination.
Conclusions
The results from this study provided evidence of in vivo function of C. viscosum as a tick repellent in accordance with a scientific-based traditional medicine of Nepali culture. These results identified potential target bioactive compounds that might be used as natural chemical combinations for the design of protective drugs or repellents in tick-rich environments, especially in rural areas in which health and economic resources are limited. Future research is needed related to concentrations and toxicity properties of C. viscosum extracts and their principal components. This study provided in vivo demonstration of repellent activity from a leave extract and elucidated additional information of the natural composition to support indigenous medicine and arise further exploration of new and safe biological products and applications for the development of tick repellents based on natural sources.
Study rationale and experimental design. (A) In the Nepali Chitwan National Park, indigenous medicine uses extracts from Clerodendrum viscosum (Bhatti) and Mangifera indica (mango) plant leave extracts to prevent ticks from invading or to remove them from the ear canal. Nepalis construct a funnel with leaves, place it on the ear canal and add a burning coal piece to extract plant leaf juice with repellent activity. (B) Based on this information, we collected at the Nepali Chitwan National Park C. viscosum leaves and flowers together with mango leaves also associated with repellent activity to characterize their effect on ticks under laboratory conditions. Photographs were taken on Chitwan National Park by researchers
Evaluation of tick repellent activity of plant extract. Plant extracts were prepared from Clerodendrum viscosum (Bhatti) leaves and flowers and Mangifera indica (mango) leaves and used to evaluate tick repellency. (A) Petri dish repellency bioassay. A filter paper with an open hole in the center was placed in the Petri dish. Plant extracts or phosphate-buffered saline (PBS, control) were applied to different sides of the paper and after 5 min, 10 unfed questing Ixodes ricinus ticks (1:1 female:male) were added in the dish center. Tick counts after 10 min were recorded and used to evaluate repellency by comparison between both sides of the dish and groups combined by plant components (one-way ANOVA followed by Tukey HSD test and Bonferroni–Holm method; n = 4 biological replicates). (B) To provide additional support, a single trial was conducted under tick climbing repellency bioassay conditions. Plant extracts or PBS were added to filter paper strips and placed inside 15-ml Corning tubes. After 5 min, 10 unfed questing I. ricinus ticks (1:1 female:male) were added to the bottom of the tube. Ten min later, the percentage of ticks climbing on the filter papers was calculated and compared between groups
References
Adenubi OT, Ahmed AS, Fasina FO et al (2018a) Pesticidal plants as a possible alternative to synthetic acaricides in tick control: a systematic review and meta-analysis. Ind Crops Prod 123:779–806. https://doi.org/10.1016/j.indcrop.2018.06.075
Adenubi OT, McGaw LJ, Eloff JN, Naidoo V (2018b) In vitro bioassays used in evaluating plant extracts for tick repellent and acaricidal properties: a critical review. Vet Parasitol 254:160–171. https://doi.org/10.1016/j.vetpar.2018.03.008
Alain A, Hamidou CF, Louise A et al (2022) Plants used in Côte d’Ivoire (West Africa) against ticks: evaluation for acaricidal activity against Rhipicephalus (Boophilus) microplus. Vet Parasitol Reg Stud Rep 35:100780. https://doi.org/10.1016/j.vprsr.2022.100780
Alwala OJ, Wanzala W, Inyambukho RA et al (2010) Characterization and evaluation of Repellent Effect of essential oil of Mangifera indica L. from Kenya. J Essent Oil Bear Plants 13:85–96. https://doi.org/10.1080/0972060X.2010.10643795
Amin MR, Mondol R, Habib MR, Hossain MT (2012) Antimicrobial and cytotoxic activity of three bitter plants Enhydra fluctuans, Andrographis peniculata and clerodendrum viscosum. Adv Pharm Bull 2:207–211. https://doi.org/10.5681/apb.2012.032
Bhattacharjee PP, Ray D (2010) Pest management beliefs and practices of Manipuri rice farmers in Barak Valley, Assam. Indian J Tradit Knowl 9:673–676
Bhattarai S, Chaudhary RP, Quave CL, Taylor RSL (2010) The use of medicinal plants in the trans-himalayan arid zone of Mustang district, Nepal. J Ethnobiol Ethnomedicine 6:14. https://doi.org/10.1186/1746-4269-6-14
Bird GWG (1961) Hæmagglutinins from Clerodendrum viscosum Vent. Nature 191:292–292. https://doi.org/10.1038/191292a0
Cakabay T, Gokdogan O, Kocyigit M (2016) Human otoacariasis: demographic and clinical outcomes in patients with ear-canal ticks and a review of literature. J Otol 11:111–117. https://doi.org/10.1016/j.joto.2016.06.003
Chen J, Chen J-J, Gao K (2014) Chemical constituents and biological activities of Dicranopteris linearis. Chem Nat Compd 49:1129–1132
Coppola ED, Conrad EC, Cotter R (1978) High pressure liquid chromatographic determination of major organic acids in cranberry juice. J - Assoc Off Anal Chem 61:1490–1492
Dantas ACS, Machado DMR, Araujo AC et al (2015) Acaricidal activity of extracts from the leaves and aerial parts of Neoglaziovia variegata (Bromeliaceae) on the cattle tick Rhipicephalus (Boophilus) microplus. Res Vet Sci 100:165–168. https://doi.org/10.1016/j.rvsc.2015.04.012
Dautel H (2004) Test systems for tick repellents. Int J Med Microbiol IJMM 293 Suppl 37:182–188. https://doi.org/10.1016/s1433-1128(04)80037-8
de la Fuente J (2018) Controlling ticks and tick-borne diseases… looking forward. Ticks Tick-Borne Dis 9:1354–1357. https://doi.org/10.1016/j.ttbdis.2018.04.001
Doğan M, Devge C, Tanrıöver O et al (2012) Facial nerve paralysis due to intra-aural Hyalomma tick infestation. Turk Parazitolojii Derg 36:254–257. https://doi.org/10.5152/tpd.2012.60
Fegan D, Glennon J (1996) Intra-aural ticks in Nepal. Lancet Lond Engl 348:1313. https://doi.org/10.1016/S0140-6736(05)65794-7
Felipe DF, Brambilla LZS, Porto C et al (2014) Phytochemical analysis of Pfaffia glomerata inflorescences by LC-ESI-MS/MS. Molecules 19:15720–15734. https://doi.org/10.3390/molecules191015720
Fernández-Poyatos M, del Ruiz-Medina P, Zengin A, Llorent-Martínez G EJ (2019) Phenolic characterization, antioxidant activity, and enzyme Inhibitory Properties of Berberis thunbergii DC. Leaves: a Valuable source of phenolic acids. Molecules 24:4171. https://doi.org/10.3390/molecules24224171
Ghosh G, Panda P, Rath M et al (2015) GC-MS analysis of bioactive compounds in the methanol extract of Clerodendrum viscosum leaves. Pharmacogn Res 7:110–113. https://doi.org/10.4103/0974-8490.147223
Gliniewicz A, Mikulak E, Przygodzka M (2017) Methods of testing repellent efficiency against ticks. Przegl Epidemiol 71:457–465
Graber CD, Goust JM, Glassman AD, Kendall R, Loadholt CB (1981) Immunomodulating properties of dimethylglycine in humans. J Infect Dis 143(1):101–105. https://doi.org/10.1093/infdis/143.1.101
Gray JS, Kahl O, Lane RS et al (2016) Diapause in ticks of the medically important Ixodes ricinus species complex. Ticks Tick-Borne Dis 7:992–1003. https://doi.org/10.1016/j.ttbdis.2016.05.006
Gross AD, Temeyer KB, Day TA et al (2017) Interaction of plant essential oil terpenoids with the southern cattle tick tyramine receptor: a potential biopesticide target. Chem Biol Interact 263:1–6. https://doi.org/10.1016/j.cbi.2016.12.009
Gupta M, Raut R, Manandhar S et al (2023) Identification and characterization of probiotics isolated from indigenous chicken (Gallus domesticus) of Nepal. PLoS ONE 18:e0280412. https://doi.org/10.1371/journal.pone.0280412
Han W-M, Chen X-C, Li G-R, Wang Y (2020) Acacetin protects against high Glucose-Induced endothelial cells Injury by preserving mitochondrial function via activating Sirt1/Sirt3/AMPK signals. Front Pharmacol 11:607796. https://doi.org/10.3389/fphar.2020.607796
Hartmann D, Šíma R, Konvičková J et al (2018) Multiple legumain isoenzymes in ticks. Int J Parasitol 48:167–178. https://doi.org/10.1016/j.ijpara.2017.08.011
Heatherbell DA, Struebi P, Eschenbruch R, Withy LM (1980) A New Fruit Wine from Kiwifruit: a wine of unusual composition and Riesling Sylvaner Character. Am J Enol Vitic 31:114–121
Heikkilä E, Hermant A, Thevenet J et al (2019) The plant product quinic acid activates Ca2+-dependent mitochondrial function and promotes insulin secretion from pancreatic beta cells. Br J Pharmacol 176:3250–3263. https://doi.org/10.1111/bph.14757
Huang Q, Ma Q, Li F et al (2022) Metabolomics reveals changes in Metabolite Profiles among Pre-Diapause, Diapause and Post-Diapause Larvae of Sitodiplosis mosellana (Diptera: Cecidomyiidae). Insects 13:339. https://doi.org/10.3390/insects13040339
Huynh OA, Jankowicz-Cieslak J, Saraye B et al (2017) Low-cost methods for DNA extraction and quantification. In: Jankowicz-Cieslak J, Tai TH, Kumlehn J, Till BJ (eds) Biotechnologies for Plant Mutation breeding: protocols. Springer International Publishing, Cham, pp 227–239
Indudharan R, Ahamad M, Ho TM et al (1999) Human otoacariasis. Ann Trop Med Parasitol 93:163–167. https://doi.org/10.1080/00034989958645
Ishtiaq M, Maqbool M, Ajaib M et al (2021) Ethnomedicinal and folklore inventory of wild plants used by rural communities of valley Samahni, District Bhimber Azad Jammu and Kashmir, Pakistan. PLoS ONE 16:e0243151. https://doi.org/10.1371/journal.pone.0243151
Joshi N, Ghorbani A, Siwakoti M, Kehlenbeck K (2020) Utilization pattern and indigenous knowledge of wild medicinal plants among three ethnic groups in Makawanpur district, Central Nepal. J Ethnopharmacol 262:113219. https://doi.org/10.1016/j.jep.2020.113219
Khan S, Somerville D, Frese M, Nayudu M (2020) Environmental gut bacteria in european honey bees (Apis mellifera) from Australia and their relationship to the chalkbrood disease. PLoS ONE 15:e0238252. https://doi.org/10.1371/journal.pone.0238252
Kubo I, Chen Q-X, Nihei K (2003) Molecular design of antibrowning agents: antioxidative tyrosinase inhibitors. Food Chem 81:241–247. https://doi.org/10.1016/S0308-8146(02)00418-1
Kularatne SAM, Fernando R, Selvaratnam S et al (2018) Intra-aural tick bite causing unilateral facial nerve palsy in 29 cases over 16 years in Kandy, Sri Lanka: is rickettsial aetiology possible? BMC Infect Dis 18:418. https://doi.org/10.1186/s12879-018-3338-8
Kwak ML, Chavatte J-M, Chew KL, Lee BPY-H (2021) Emergence of the zoonotic tick Dermacentor (Indocentor) auratus Supino, 1897 (Acari: Ixodidae) in Singapore. Ticks Tick-Borne Dis 12:101574. https://doi.org/10.1016/j.ttbdis.2020.101574
Lee X, Wong C, Coats J, Paskewitz S (2022) Field evaluations of three botanically inspired repellents against the Blacklegged Tick, Ixodes scapularis (Acari: Ixodidae). https://doi.org/10.1093/jme/tjac111. J Med Entomol tjac111
Li TT, Gu CT (2022) Lactobacillus huangpiensis sp. nov. and Lactobacillus laiwuensis sp. nov., isolated from the gut of honeybee (Apis mellifera). Int J Syst Evol Microbiol 72. https://doi.org/10.1099/ijsem.0.005237
Liu C, Zhang M, Ye S et al (2021) Acacetin protects myocardial cells against Hypoxia-Reoxygenation Injury through activation of Autophagy. J Immunol Res 2021:9979843. https://doi.org/10.1155/2021/9979843
Liu Y, Wang X, Luo S et al (2022) Metabolomic and transcriptomic analyses identify quinic acid protecting eggplant from damage caused by western flower thrips. Pest Manag Sci 78:5113–5123. https://doi.org/10.1002/ps.7129
Llorent-Martínez EJ, Spínola V, Gouveia S, Castilho PC (2015) HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Ind Crops Prod 69:80–90. https://doi.org/10.1016/j.indcrop.2015.02.014
Lopes SP, Yepes LM, Pérez-Castillo Y et al (2020) Alkyl and Aryl Derivatives Based on p-Coumaric Acid Modification and Inhibitory Action against Leishmania braziliensis and Plasmodium falciparum. Mol Basel Switz 25:E3178. https://doi.org/10.3390/molecules25143178
Luitel DR, Rokaya MB, Timsina B, Münzbergová Z (2014) Medicinal plants used by the Tamang community in the Makawanpur district of central Nepal. J Ethnobiol Ethnomedicine 10:5. https://doi.org/10.1186/1746-4269-10-5
Luns DAR, Soares L, de Guedes S NA, et al (2021) Bioactivity of Meliaceae, Amaryllidaceae, Solanaceae and Amaranthaceae plant aqueous extracts against the cattle tick Rhipicephalus microplus. Nat Prod Res 1–5. https://doi.org/10.1080/14786419.2021.2016744
Malarz J, Michalska K, Galanty A et al (2023) Constituents of Pulicaria inuloides and cytotoxic activities of two Methoxylated Flavonols. Molecules 28:480. https://doi.org/10.3390/molecules28020480
Mateos-Hernández L, Obregón D, Maye J et al (2020) Anti-Tick Microbiota Vaccine Impacts Ixodes ricinus performance during feeding. Vaccines 8:E702. https://doi.org/10.3390/vaccines8040702
Mawela KG, Luseba D, Magano S, Eloff JN (2019) Repellent properties of Rotheca glabrum plant extracts against adults of Rhipicephalus appendiculatus. BMC Vet Res 15:122. https://doi.org/10.1186/s12917-019-1853-5
Nandi S, Lyndem LM (2016) Clerodendrum viscosum: traditional uses, pharmacological activities and phytochemical constituents. Nat Prod Res 30:497–506. https://doi.org/10.1080/14786419.2015.1025229
Nieto-Peñalver CG, Savino MJ, Bertini EV et al (2014) Gluconic acid produced by Gluconacetobacter diazotrophicus Pal5 possesses antimicrobial properties. Res Microbiol 165:549–558. https://doi.org/10.1016/j.resmic.2014.06.003
Nwanade CF, Wang M, Wang T et al (2020) Botanical acaricides and repellents in tick control: current status and future directions. Exp Appl Acarol 81:1–35. https://doi.org/10.1007/s10493-020-00489-z
Ogawa M, Shirasago Y, Ando S et al (2018) Caffeic acid, a coffee-related organic acid, inhibits infection by severe fever with thrombocytopenia syndrome virus in vitro. J Infect Chemother 24:597–601. https://doi.org/10.1016/j.jiac.2018.03.005
Oliveira DF, Costa VA, Terra WC et al (2019) Impact of phenolic compounds on Meloidogyne incognita in vitro and in tomato plants. Exp Parasitol 199:17–23. https://doi.org/10.1016/j.exppara.2019.02.009
Olthof MR, Hollman PC, Katan MB (2001) Chlorogenic acid and caffeic acid are absorbed in humans. J Nutr 131:66–71. https://doi.org/10.1093/jn/131.1.66
Oly WT, Islam W, Hassan P, Parween S (2011) Antimicrobial activity of Clerodendrum viscosum (Verbenaceae). Int J Agric Biol Pak
Pantoja Pulido KD, Colmenares Dulcey AJ, Isaza Martínez JH (2017) New caffeic acid derivative from Tithonia diversifolia (Hemsl.) A. Gray butanolic extract and its antioxidant activity. Food Chem Toxicol 109:1079–1085. https://doi.org/10.1016/j.fct.2017.03.059
Panzuto M, Mauffette Y, Alber PJ (2002) Developmental, gustatory, and behavioral responses of leafroller larvae, Choristoneura rosaceana, to tannic acid and glucose. J Chem Ecol 28:145–160. https://doi.org/10.1023/a:1013571020783
Park S-M, Kang J-H, Son H-J et al (2016) Combined treatments of chestnut shell extract, fumaric acid, and mild heat to inactivate foodborne pathogens inoculated on beetroot (Beta vulgaris L.) leaves. Food Sci Biotechnol 25:1217–1220. https://doi.org/10.1007/s10068-016-0193-5
Pei K, Ou J, Huang J, Ou S (2016) p-Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J Sci Food Agric 96:2952–2962. https://doi.org/10.1002/jsfa.7578
Pun SK, Guglielmone AA, Tarragona EL et al (2018) Ticks (Acari: Ixodidae) of Nepal: first record of Amblyomma varanense (Supino), with an update of species list. Ticks Tick-Borne Dis 9:526–534. https://doi.org/10.1016/j.ttbdis.2018.01.010
Quadros DG, Johnson TL, Whitney TR et al (2020) Plant-Derived Natural Compounds for Tick Pest Control in Livestock and Wildlife: pragmatism or Utopia? Insects 11:E490. https://doi.org/10.3390/insects11080490
Rajakaruna N, Harris CS, Towers GHN (2002) Antimicrobial activity of plants collected from Serpentine Outcrops in Sri Lanka. Pharm Biol 40:235–244. https://doi.org/10.1076/phbi.40.3.235.5825
Ramos B, Miller FA, Brandão TRS et al (2013) Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov Food Sci Emerg Technol 20:1–15. https://doi.org/10.1016/j.ifset.2013.07.002
Riaz M, Ahmad R, Rahman NU et al (2020) Traditional uses, Phyto-chemistry and pharmacological activities of Tagetes patula L. J Ethnopharmacol 255:112718. https://doi.org/10.1016/j.jep.2020.112718
Saha S, Mukherjee A, Biswas S et al (2018) Formulation and chemical characterization of Clerodendrum infortunatum leaf extract in relation to anti-fungal activity. Heliyon 4:e01047. https://doi.org/10.1016/j.heliyon.2018.e01047
Salaheen S, Nguyen C, Hewes D, Biswas D (2014) Cheap extraction of antibacterial compounds of berry pomace and their mode of action against the pathogen Campylobacter jejuni. Food Control
Salehi B, Albayrak S, Antolak H et al (2018) Aloe Genus plants: from farm to Food Applications and Phytopharmacotherapy. Int J Mol Sci 19:E2843. https://doi.org/10.3390/ijms19092843
Samuel R, Pathalam G, Babu V et al (2023) Biocontrol efficacy of apigenin isolated from Anisomeles indica (L.) Kuntze against immature stages of Culex quinquefasciatus (say, 1823) and its in silico studies. Biocatal Agric Biotechnol 48:102637. https://doi.org/10.1016/j.bcab.2023.102637
Schubert F, Pålsson K, Santangelo E, Borg-Karlson A-K (2017) Sulfate turpentine: a resource of tick repellent compounds. Exp Appl Acarol 72:291–302. https://doi.org/10.1007/s10493-017-0145-7
Sharma AK, Tiwari SS, Kumar S et al (2022) Establishment of antitick efficacy of a phytoformulation prepared from Annona squamosa leaf extracts for the management of acaricide resistant tick infestations on cattle. Acta Trop 233:106463. https://doi.org/10.1016/j.actatropica.2022.106463
Shen H, Yamashita A, Nakakoshi M et al (2013) Inhibitory effects of caffeic acid phenethyl ester derivatives on replication of hepatitis C virus. PLoS ONE 8:e82299. https://doi.org/10.1371/journal.pone.0082299
Shendge AK, Basu T, Chaudhuri D et al (2017) In vitro antioxidant and antiproliferative activities of various solvent fractions from Clerodendrum viscosum Leaves. Pharmacogn Mag 13:S344–S353. https://doi.org/10.4103/pm.pm_395_16
Shendge AK, Basu T, Mandal N (2021a) Evaluation of anticancer activity of Clerodendrum viscosum leaves against breast carcinoma. Indian J Pharmacol 53:377–383. https://doi.org/10.4103/ijp.IJP_565_19
Shendge AK, Chaudhuri D, Basu T, Mandal N (2021b) A natural flavonoid, apigenin isolated from Clerodendrum viscosum leaves, induces G2/M phase cell cycle arrest and apoptosis in MCF-7 cells through the regulation of p53 and caspase-cascade pathway. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex 23:718–730. https://doi.org/10.1007/s12094-020-02461-0
Simirgiotis MJ, Benites J, Areche C, Sepúlveda B (2015) Antioxidant capacities and analysis of Phenolic Compounds in three endemic Nolana Species by HPLC-PDA-ESI-MS. Mol Basel Switz 20:11490–11507. https://doi.org/10.3390/molecules200611490
Singh VK, Ali ZA, Siddiqui MK (1997) Medicinal plants used by the Forest Ethnics of Gorakhpur District (Uttar Pradesh), India. Int J Pharmacogn 35:194–206. https://doi.org/10.1076/phbi.35.3.194.13298
Sinha NK, Seth KK, Pandey VB et al (1981) Flavonoids from the flowers of Clerodendron infortunatum. Planta Med 42:296–298. https://doi.org/10.1055/s-2007-971645
Somayaji KSG, Rajeshwari A (2007) Human otoacariasis. Indian J Otolaryngol Head Neck Surg Off Publ Assoc Otolaryngol India 59:237–239. https://doi.org/10.1007/s12070-007-0069-3
Sonowal R, Nandimath K, Kulkarni SS et al (2013) Hydrolysis of aromatic β-glucosides by non-pathogenic bacteria confers a chemical weapon against predators. Proc Biol Sci 280:20130721. https://doi.org/10.1098/rspb.2013.0721
Soutar O, Cohen F, Wall R (2019) Essential oils as tick repellents on clothing. Exp Appl Acarol 79:209–219. https://doi.org/10.1007/s10493-019-00422-z
Srivastava M, Maurya P, Jyotshna, Shanker K (2021) Clerodendrum viscosum: a critical review on phytochemistry, pharmacology, quality assurance, and safety data. Med Chem Res 30:2145–2167. https://doi.org/10.1007/s00044-021-02804-8
Tabari MA, Youssefi MR, Maggi F, Benelli G (2017) Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae). Vet Parasitol 245:86–91. https://doi.org/10.1016/j.vetpar.2017.08.012
Uddin MJ, Çiçek SS, Willer J et al (2020) Phenylpropanoid and flavonoid glycosides from the leaves of Clerodendrum infortunatum (Lamiaceae). Biochem Syst Ecol 92:104131. https://doi.org/10.1016/j.bse.2020.104131
Wang G-F, Shi L-P, Ren Y-D et al (2009) Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antiviral Res 83:186–190. https://doi.org/10.1016/j.antiviral.2009.05.002
Wang HV, Pickett LJ, Faraone N (2022) Repellent and acaricidal activities of basil (Ocimum basilicum) essential oils and rock dust against Ixodes scapularis and Dermacentor variabilis ticks. Exp Appl Acarol 86:583–598. https://doi.org/10.1007/s10493-022-00705-y
Wu Y, Song F, Li Y et al (2021) Acacetin exerts antioxidant potential against atherosclerosis through Nrf2 pathway in apoE-/- mice. J Cell Mol Med 25:521–534. https://doi.org/10.1111/jcmm.16106
Yan X, Qi M, Li P et al (2017) Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci 7:50. https://doi.org/10.1186/s13578-017-0179-x
Yun S-M, Lee W-G, Ryou J et al (2014) Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg Infect Dis 20:1358–1361. https://doi.org/10.3201/eid2008.131857
Zhang X, Xu C, Liu Y et al (2020) Enhancement of glucaric acid production in Saccharomyces cerevisiae by expressing Vitreoscilla hemoglobin. Biotechnol Lett 42:2169–2178. https://doi.org/10.1007/s10529-020-02966-2
Zhang X-L, Deng Y-P, Yang T et al (2022) Metagenomics of the midgut microbiome of Rhipicephalus microplus from China. Parasit Vectors 15:48. https://doi.org/10.1186/s13071-022-05161-6
Acknowledgements
We thank support by the Center for Molecular Dynamics Nepal (CMDN), Nepal and our guider at the Chitwan National Park, Ravi Shrestha, for introducing us to the Nepali tradition related to the Clerodendrum viscosum tick repellent.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
Lorena Mazuecos: performed research and experiments, analysed data and formal analysis, writing-reviewing and editing the final manuscript. Marinela Contreras: methodology design, performed research, analysed data and writing. Paul D. Kasaija: performed research and data acquisition. Karelia Deulofeu: plant and data acquisition. Weronika Grąźlewska: performed experiments. Isabel Fernandez-Moratalla: performed research and formal analysis. Eduardo Guisantes-Batan and Sergio Gomez Alonso: designed and performed de HPLC-ESI-QToF analysis. Rajesh Man Rajbhandari: material and data acquisition. Daniel Sojka: material and data acquisition. Prajwol Manandhar: material and data acquisition and formar analysis. Libor Grubhoffer: material and data acquisition. Dibesh Karmacharya: material and data acquisition. Christian Gortazar: designed, data acquisition and supervised research. José de la Fuente: conceptualization, organized, supervised research, data acquisition, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mazuecos, L., Contreras, M., Kasaija, P.D. et al. Natural Clerodendrum-derived tick repellent: learning from Nepali culture. Exp Appl Acarol 90, 83–98 (2023). https://doi.org/10.1007/s10493-023-00804-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-023-00804-4