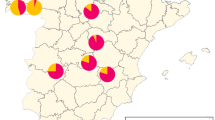
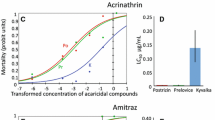
Abstract
Varroa destructor is one of the greatest threats for the European honeybee, Apis mellifera. Acaricides are required to control mite infestation. Three conventional chemical acaricide substances are used in France: tau-fluvalinate, flumethrin and amitraz. Tau-fluvalinate was used for over 10 years before experiencing a loss of effectiveness. In 1995, bioassay trials showed the first mite resistance to tau-fluvalinate. In some countries, amitraz was widely used, also leading to resistance of V. destructor to amitraz. In France, some efficiency field tests showed a loss of treatment effectiveness with amitraz. We adapted the bioassay from Maggi and collaborators to determine mite susceptibility to tau-fluvalinate and amitraz in France in 2018 and 2019. The lethal concentration (LC) which kills 90% of susceptible mite strains (LC90) is 0.4 and 12 µg/mL for amitraz and tau-fluvalinate, respectively. These concentrations were chosen as the determining factors to evaluate mite susceptibility. Some mites, collected from different apiaries, present resistance to amitraz and tau-fluvalinate (71% of the mite samples show resistance to amitraz and 57% to tau-fluvalinate). As there are few active substances available in France, and if mite resistance to acaricides continues to increase, the effectiveness of the treatments will decrease and therefore more treatments per year will be necessary. To prevent this situation, a new strategy needs to be put in place to include mite resistance management. We suggest that a bioassay would be a good tool with which to advise the policymakers.



Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Adjlane H, Noureddine N (2017) Evaluation of the resistance of the mite Varroa destructor to the amitraz in colonies of honey bees (Apis mellifera) in Algeria. Uludağ Arıcılık Dergisi 17(1):1–6. https://doi.org/10.31467/uluaricilik.373716
Alissandrakis E, Ilias A, Tsagkarakou A (2017) Pyrethroid target site resistance in Greek populations of the honey bee parasite Varroa destructor (Acari: Varroidae). J Apic Res 56(5):625–630. https://doi.org/10.1080/00218839.2017.1368822
Anderson DL, Trueman JWH (2000) Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp Appl Acarol 24(3):165–189. https://doi.org/10.1023/A:1006456720416
Baron S, Barrero RA, Black M, Bellgard MI, van Dalen EMS, Fourie J, Maritz-Olivier C (2018) Differentially expressed genes in response to amitraz treatment suggests a proposed model of resistance to amitraz in R. decoloratus ticks. Int J Parasitol Drugs Drug Resist 8(3):361–371
Baron S, van der Merwe NA, Madder M, Maritz-Olivier C, Munderloh UG (2015) SNP analysis infers that recombination is involved in the evolution of amitraz resistance in Rhipicephalus microplus. PLOS ONE 10(7):e0131341
Baude M, Kunin WE, Boatman NDB, Conyers S, Davies N, Gillespie MAK, Morton RD, Smart SM, Memmott J (2016) Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 530(7588):85–88. https://doi.org/10.1038/nature16532
Beaurepaire AL, Krieger KJ, Moritz RFA (2017) Seasonal cycle of inbreeding and recombination of the parasitic mite Varroa destructor in honeybee colonies and its implications for the selection of acaricide resistance. Infect Genet Evolut 50:49–54. https://doi.org/10.1016/j.meegid.2017.02.011
Beaurepaire A, Piot N, Doublet V, Antunez K, Campbell E, Chantawannakul P, Chejanovsky N et al (2020) Diversity and global distribution of viruses of the western honey bee, Apis mellifera. Insects 11(4):239. https://doi.org/10.3390/insects11040239
Bianchi MW, Barré N, Messad N (2003) Factors related to cattle infestation level and resistance to acaricides in Boophilus Microplus tick populations in New Caledonia. Vet Parasitol 112(1–2):75–89. https://doi.org/10.1016/S0304-4017(02)00415-6
Brattsten LB, Holyoke CW, Leeper JR, Raffa KF (1986) Insecticide resistance: challenge to pest management and basic research. Science 231(4743):1255–1260. https://doi.org/10.1126/science.231.4743.1255
Cervo R, Bruschini C, Cappa F, Meconcelli S, Pieraccini G, Pradella D, Turillazzi S (2014) High varroa mite abundance influences chemical profiles of worker bees and mite–host preferences. J Exp Biol 217(17):2998–3001. https://doi.org/10.1242/jeb.099978
Cheng X, Umina PA, Lee SF, Hoffmann AA (2019) Pyrethroid resistance in the pest mite, Halotydeus destructor: dominance patterns and a new method for resistance screening. Pestic Biochem Physiol 159:9–16. https://doi.org/10.1016/j.pestbp.2019.04.010
Chevillon C, Ducornez S, de Meeûs T, Koffi BB, Gaïa H, Delathière JM, Barré N (2007) Accumulation of acaricide resistance mechanisms in Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) populations from New Caledonia Island. Vet Parasitol 147(3):276–288. https://doi.org/10.1016/j.vetpar.2007.05.003
Cossío-Bayúgar R, Martínez-Ibañez F, Aguilar-Díaz H, Miranda E (2018) Pyrethroid acaricide resistance is proportional to P-450 cytochrome oxidase expression in the cattle tick Rhipicephalus microplus Research Article. Biomed Res Int. https://doi.org/10.1155/2018/8292465
Denholm I (1998) Challenges with managing insecticide resistance in agricultural pests, exemplisfied by the whitefly Bemisia tabaci. Philos Trans R Soc B 353(1376):1757–1767. https://doi.org/10.1098/rstb.1998.0328
Dietemann V, Nazzi F, Martin SJ, Anderson DL, Locke B, Delaplane KS, Wauquiez Q et al (2013) Standard methods for varroa research. J Apic Res 52(1):1–54. https://doi.org/10.3896/IBRA.1.52.1.09
Dietemann V, Beaurepaire A, Page P, Yañez O, Buawangpong N, Chantawannakul P, Neumann P (2019) Population genetics of ectoparasitic mites Varroa spp. in eastern and western honey bees. Parasitology. https://doi.org/10.1017/S003118201900091X
Dmitryjuk M, Żółtowska K, Frączek R, Lipiński Z (2014) Esterases of Varroa destructor (Acari: Varroidae), parasitic mite of the honeybee. Exp Appl Acarol 62(4):499–510. https://doi.org/10.1007/s10493-013-9754-y
Dutta S, Godara R, Katoch R, Yadav A, Katoch M, Singh NK (2017) Detection of amitraz and malathion resistance in field populations of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in Jammu region of India. Exp Appl Acarol 71(3):291–301
Dynes TL, De Roode JC, Lyons JI, Berry JA, Delaplane KS, Brosi BJ (2017) Fine scale population genetic structure of Varroa destructor, an ectoparasitic mite of the honey bee (Apis Mellifera). Apidologie 48(1):93–101. https://doi.org/10.1007/s13592-016-0453-7
Elzen P, Westervelt D (2004) A scientific note on reversion of fluvalinate resistance to a degree of susceptibility in Varroa Destructor. Apidologie 35(5):519–520. https://doi.org/10.1051/apido:2004036
Elzen PJ, Eischen FA, Baxter JB, Pettis J, Elzen GW, Wilson WT (1998) Fluvalinate resistance in Varroa jacobsoni from several geographic locations. American Bee J (USA). https://agris.fao.org/agris-search/search.do?recordID=US1997079119
Elzen PJ, Eischen FA, Baxter JR, Elzen GW, Wilson WT (1999) Detection of resistance in US Varroa jacobsoni Oud. (Mesostigmata: Varroidae) to the acaricide fluvalinate. Apidologie 30(1):13–17. https://doi.org/10.1051/apido:19990102
Fakhimzadeh K (2001) Effectiveness of confectioner sugar dusting to knock down Varroa destructor from adult honey bees in laboratory trials. Apidologie 32(2):139–148. https://doi.org/10.1051/apido:2001119
FAO (2013) Directives pour la prévention et la gestion de la résistance aux pesticides, Organisation des Nations Unies pour l’alimentation et l’agriculture (FAO). http://www.fao.org/3/a-bt561f.pdf.
Fries I, Imdorf A, Rosenkranz P (2006) Survival of mite infested (Varroa destructor) honey bee (Apis mellifera) colonies in a nordic climate. Apidologie 37(5):564–570. https://doi.org/10.1051/apido:2006031
Gisder S, Aumeier P, Genersch E (2009) Deformed wing virus: replication and viral load in mites (Varroa destructor). J Gen Virol 90(2):463–467. https://doi.org/10.1099/vir.0.005579-0
González-Cabrera J, Davies TGE, Field LM, Kennedy PJ, Williamson MS (2013) An amino acid substitution (L925V) Associated with resistance to pyrethroids in Varroa destructor. PLoS ONE 8(12):e82941. https://doi.org/10.1371/journal.pone.0082941
González-Cabrera J, Rodríguez-Vargas S, Davies TGE, Field LM, Schmehl D, Ellis JD, Krieger K, Williamson MS (2016) Novel mutations in the voltage-gated sodium channel of pyrethroid-resistant Varroa destructor populations from the Southeastern USA. PLoS ONE 11(5):e0155332. https://doi.org/10.1371/journal.pone.0155332
González-Cabrera J, Bumann H, Rodríguez-Vargas S, Kennedy PJ, Krieger K, Altreuther G, Hertel, Nauen R, Williamson MS (2018) A single mutation is driving resistance to pyrethroids in European populations of the parasitic mite, Varroa destructor. J Pest Sci 91(3):1137–1144. https://doi.org/10.1007/s10340-018-0968-y
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347(6229):1255957. https://doi.org/10.1126/science.1255957
Grab H, Branstetter MG, Amon N, Urban-Mead KR, Park MG, Gibbs J, Blitzer EJ, Poveda K, Loeb G, Danforth BN (2019) Agriculturally dominated landscapes reduce bee phylogenetic diversity and pollination services. Science 363(6424):282–284. https://doi.org/10.1126/science.aat6016
Greatti M, Milani N, Nazzi F (1992) Reinfestation of an acaricide-treated apiary by Varroa jacobsoni Oud. Exp Appl Acarol 16(4):279–286. https://doi.org/10.1007/BF01218569
Gregorc A, Alburaki M, Sampson B, Knight PR, Adamczyk J (2018) Toxicity of selected acaricides to honey bees (Apis mellifera) and varroa (Varroa destructor Anderson and Trueman) and their use in controlling varroa within honey bee colonies. Insects 9(2):20. https://doi.org/10.3390/insects9020055
Hillesheim E, Ritter W, Bassand D (1996) First data on resistance mechanisms of Varroa jacobsoni (OUD) against tau-fluvalinate. Exp Appl Acarol 20(5):283–296. https://doi.org/10.1007/BF00052878
Ilias A, Vontas J, Tsagkarakou A (2014) Global distribution and origin of target site insecticide resistance mutations in Tetranychus urticae. Insect Biochem Mol Biol 48:17–28
Jonsson NN, Hope M (2007) Progress in the epidemiology and diagnosis of amitraz resistance in the cattle tick Boophilus microplus. Vet Parasitol 146(3):193–198. https://doi.org/10.1016/j.vetpar.2007.03.006
Kamler M, Nesvorna M, Stara J, Erban T, Hubert J (2016) Comparison of tau-fluvalinate, acrinathrin, and amitraz effects on susceptible and resistant populations of Varroa destructor in a vial test. Exp Appl Acarol 69(1):1–9. https://doi.org/10.1007/s10493-016-0023-8
Kanga Lambert HB, Adamczyk J, Marshall K, Cox R (2010) Monitoring for resistance to organophosphorus and pyrethroid insecticides in Varroa mite populations. J Econ Entomol 103(5):1797–1802. https://doi.org/10.1603/EC10064
Klafke G, Webster A, Agnol BD, Pradel E, Silva J, de La Canal LH, Becker M et al (2017) Multiple resistance to acaricides in field populations of Rhipicephalus microplus from Rio Grande Do Sul State Southern Brazil. Ticks Tick-Borne Dis 8(1):73–80. https://doi.org/10.1016/j.ttbdis.2016.09.019
Li AY, Davey RB, Miller RJ, George JE (2004) Detection and characterization of amitraz resistance in the Southern Cattle Tick, Boophilus microplus (Acari: Ixodidae). J Med Entomol 41(2):193–200. https://doi.org/10.1603/0022-2585-41.2.193
Loope K, Baty JW, Lester PJ, Wilson R (2019) Pathogen shifts in a honeybee predator following the arrival of the Varroa mite. Proc R Soc B 286:20182499. https://doi.org/10.1098/rspb.2018.2499
Losey JE, Vaughan M (2006) The Economic value of ecological services provided by insects. Bioscience 56(4):311–323. https://doi.org/10.1641/0006-3568(2006)56[311:TEVOES]2.0.CO;2
Maciel WG, Lopes WDZ, Cruz BC, Gomes LVC, Teixeira WFP, Buzzulini C, Bichuette MA et al (2015) Ten years later: Evaluation of the effectiveness of 12.5% Amitraz against a field population of Rhipicephalus (Boophilus) microplus using field studies, artificial infestation (Stall Tests) and adult immersion tests. Vet Parasitol 214(3–4):233–241. https://doi.org/10.1016/j.vetpar.2015.10.024
Maggi MD, Ruffinengo SR, Gende LB, Eguaras MJ, Sardella NH (2008) LC50 baseline levels of amitraz, coumaphos, fluvalinate and flumethrin in populations of Varroa destructor from Buenos Aires Province, Argentina. J Apic Res 47(4):292–295. https://doi.org/10.1080/00218839.2008.11101477
Maggi MD, Ruffinengo SR, Negri P, Eguaras MJ (2010) Resistance phenomena to amitraz from populations of the ectoparasitic mite Varroa destructor of Argentina. Parasitol Res 107(5):1189–1192. https://doi.org/10.1007/s00436-010-1986-8
Mathieu L, Faucon J-P (2015) Changes in the response time for exposed to amitraz. J Apic Res 39(3–4):155–158
Messan K, DeGrandi-Hoffman G, Castillo-Chavez C, Kang Y. 2017. Migration effects on population dynamics of the honeybee-mite interactions. In: Banerjee M, Perasso A, Venturino E (eds.) Mathematical modelling of natural phenomena 12 (2): 84–115. https://doi.org/10.1051/mmnp/201712206
Milani N (1995) The resistance of Varroa jacobsoni Oud to pyrethroids: a laboratory assay. Apidologie 26(5):415–429. https://doi.org/10.1051/apido:19950507
Milani N, Vedova GD (2002) Decline in the proportion of mites resistant to fluvalinate in a population of Varroa destructor not treated with pyrethroids. Apidologie 33(4):417–422. https://doi.org/10.1051/apido:2002028
Mondet F, de Miranda JR, Kretzschmar A, Le Conte Y, Mercer AR (2014) On the front line: quantitative virus dynamics in honeybee (Apis mellifera L.) Colonies along a new expansion front of the parasite Varroa destructor. PLoS Pathog 10(8):e1004323. https://doi.org/10.1371/journal.ppat.1004323
Panini M, Reguzzi MC, Chiesa O, Cominelli F, Lupi D, Moores G, Mazzoni E (2019) Pyrethroid resistance in Italian populations of the mite Varroa destructor: a focus on the Lombardy region. Bull Insectol 72(2):227–232
Peck DT, Seeley TD (2019) Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honey bee colonies to their neighbors. PLoS ONE 14(6):e0218392. https://doi.org/10.1371/journal.pone.0218392
Peck DT, Smith ML, Seeley TD (2016) Varroa destructor mites can nimbly climb from flowers onto foraging honey bees. PLoS ONE 11(12):e0167798. https://doi.org/10.1371/journal.pone.0167798
Plapp FW, Vinson SB (1977) Comparative toxicities of some insecticides to the Tobacco Budworm and its ichneumonid parasite Campoletis sonorensis. Environ Entomol 6(3):381–384. https://doi.org/10.1093/ee/6.3.381
R Development Core Team (2005) R: a language and environment for statistical computing. R foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, https://www.R-project.org.
Rinkevich FD (2020) Detection of amitraz resistance and reduced treatment efficacy in the varroa mite, Varroa destructor, within commercial beekeeping operations. PLoS ONE 15(1):e0227264. https://doi.org/10.1371/journal.pone.0227264
Rodríguez-Dehaibes SR, Otero-Colina G, Sedas VP, Villanueva Jiménez JA (2005) Resistance to amitraz and flumethrin in Varroa destructor populations from Veracruz, Mexico. J Apic Res 44(3):124–125. https://doi.org/10.1080/00218839.2005.11101162
Rodriguez-Vivas RI, Ojeda-Chi MM, Trinidad-Martinez I, Bolio-González ME (2017) First report of amitraz and cypermethrin resistance in Rhipicephalus sanguineus sensu lato infesting dogs in Mexico. Med Vet Entomol 31(1):72–77. https://doi.org/10.1111/mve.12207
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103:96–119. https://doi.org/10.1016/j.jip.2009.07.016
Ryabov EV, Childers AK, Chen Y, Madella S, Nessa A, vanEngelsdorp D, Evans JD (2017) Recent spread of Varroa destructor virus-1, a honeybee pathogen, in the United States. Sci Rep 7(1):17447. https://doi.org/10.1038/s41598-017-17802-3
Oliver R (2017) A test of using CO2 for bee friendly mite monitoring. Am Bee J. https://scientificbeekeeping.com/a-test-of-using-co2-for-bee-friendly-mite-monitoring/
Snodgrass RE (1996) Glass-vial bioassay. To estimate insecticide resistance in adult tarnished plant bugs. J Econ Entomol 89(5):1053–1059. https://doi.org/10.1093/jee/89.5.1053
Sparks TC, Nauen R (2015) IRAC: mode of action classification and insecticide resistance management. Pestic Biochem Physiol 121:122–128
Sudo M, Takahashi D, Andow DA, Suzuki Y, Yamanaka T (2017) Optimal management strategy of insecticide resistance under various insect life histories: heterogeneous timing of selection and interpatch dispersal. Evol Appl 11(2):271–283. https://doi.org/10.1111/eva.12550
Sumpter DJT, Martin SJ (2004) The dynamics of virus epidemics in varroa-infested honey bee colonies. J Anim Ecol 73(1):51–63
Sungirai M, Baron S, Moyo DZ, De Clercq P, Maritz-Olivier C, Madder M (2018) Genotyping acaricide resistance profiles of Rhipicephalus microplus tick populations from communal land areas of Zimbabwe. Ticks Tick Borne Dis 9(1):2–9
Techer MA, Rane RV, Grau ML, Roberts JMK, Sullivan ST, Liachko I, Childers AK, Evans JD, Mikheyev AS (2019) Divergent evolutionary trajectories following speciation in two Ectoparasitic honey bee mites. Commun Biol 2(1):1–16. https://doi.org/10.1038/s42003-019-0606-0
Traynor K, Mondet F, de Miranda J, Techer M, Kowallik V, Oddie M, Chantawannakul P, McAfee A (2020) Varroa destructor: a complex parasite, crippling honeybees worldwide, 2020020374. https://doi.org/10.20944/preprints202002.0374.v1
Trouiller J (1998) Monitoring Varroa jacobsoni resistance to pyrethroids in Western Europe. Apidologie 29(6):537–546. https://doi.org/10.1051/apido:19980606
van Dooremalen C, Gerritsen L, Cornelissen B, Van der Steen JJM, Van Langevelde F, Blacquière T (2012) Winter survival of individual honey bees and honey bee colonies depends on level of Varroa destructor infestation. PLoS ONE 7(4):e36285. https://doi.org/10.1371/journal.pone.0036285
Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem Mol Biol 40(8):563–572. https://doi.org/10.1016/j.ibmb.2010.05.008
Vandame J (2019) Médicaments de lutte contre Varroa destructor: Tests d’efficacité 2018, Evolution depuis 2007. La Santé de l’Abeille, no 291:229–255
Wegener J, Ruhnke H, Scheller K, Mispagel S, Knollmann U, Kamp G, Bienefeld K (2016) Pathogenesis of varroosis at the level of the honey bee (Apis mellifera) colony. J Insect Physiol 91:1–9. https://doi.org/10.1016/j.jinsphys.2016.06.004
World Health Organization (WHO) (2013) Instruction for determining the susceptibility or resistance of mosquito larvae to insecticides. Division of Vector Biology and Control
WHO (2016) Test procedures for insecticide resistance monitoring in malaria vector mosquitos. World Health Organization. https://www.who.int/malaria/publications/atoz/9789241511575/en/
Xu D, He Y, Zhang Y, Xie W, Wu Q, Wang S (2018) Status of pesticide resistance and associated mutations in the two-spotted spider mite, Tetranychus urticae, in China. Pestic Biochem Physiol 150:89–96
Zulian G, Maes J, Paracchini ML (2013) Linking land cover data and crop yields for mapping and assessment of pollination services in Europe. Land 2(3):472–492. https://doi.org/10.3390/land2030472
Acknowledgements
We acknowledge all the beekeepers, the veterinarians and the Association of Beekeeping Development in the Auvergne-Rhône-Alpes region (ADA AURA) for their participation in this project. We also would like to thank Robin Azemar and Roman Catherin for participating in the bioassay trial. We also would like to thank the reviewers for their advice and their participation in the improvent of the clarity of this article.
Funding
This study was supported by Vita beehealth, the Research and Training center Apinov, and the National Agency of the Technology Research (ANRT). This manuscript is funded by the National Agency of Technology Research (ANRT) (n°2018/0060), Vita beehealth Europe and the Research and Training center, APINOV.
Author information
Authors and Affiliations
Contributions
Benjamin Poirot and Christelle Suppo supervised the research. Material preparation and data collection was carried out by Gabrielle Almecija, Precillia Cochard and Benjamin Poirot. Statistical analysis was collated by Gabrielle Almecija, Précillia Cochard and Christelle Suppo. All authors contributed to the redaction.
Corresponding author
Ethics declarations
Conflict of interest
Gabrielle Almecija, Précillia Cochard and Benjamin Poirot are members of Apinov. Apinov has received research grants from Vitabeehealth to provide Research and Development of Vita beehealth’s products in France. Vita beehealth sells the Apistan® product and they also funded a lot of research into the tau-fluvalinate resistance between 1995 and 2000.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almecija, G., Poirot, B., Cochard, P. et al. Inventory of Varroa destructor susceptibility to amitraz and tau-fluvalinate in France. Exp Appl Acarol 82, 1–16 (2020). https://doi.org/10.1007/s10493-020-00535-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-020-00535-w