Abstract
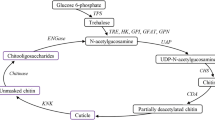
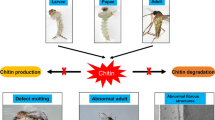
Chitinases are hydrolytic enzymes that are required for chitin degradation and reconstruction in arthropods. In this study, we report a cDNA sequence encoding a putative chitinase (PcCht1) from the citrus red mite, Panonychus citri. The PcCht1 (564 aa) possessed a signal peptide, a conserver domain, and a chitin-binding domain. Structural and phylogenetic analyses found that PcCht1 had high sequence similarity to chitinases in Tetranychus urticae. Real-time quantitative PCR analyses showed that the transcript levels of PcCht1 peaked periodically in larval and nymph stages. Moreover, significant increase of PcCht1 transcript level in the larvae was observed upon the exposure of diflubenzuron. In contrast, exposures of the larvae to diflubenzuron resulted in the decreased chitin content. Furthermore, through a feeding-based RNA interference approach, we were able to reduce the PcCht1 transcript level by 59.7 % in the larvae, and consequently the treated larvae showed a very low molting rate compared with the control. Our results expanded the understanding of the important role of PcCht1 in the growth and development of P. citri.






Similar content being viewed by others
References
Abo-Elghar GE, Fujiyoshi P, Matsumura F (2004) Significance of the sulfonylurea receptor (SUR) as the target of diflubenzuron in chitin synthesis inhibition in Drosophila melanogaster and Blattella germanica. Insect Biochem Mol Biol 34:743–752
Arakane Y, Muthukrishnan S (2010) Insect chitinase and chitinase-like proteins. Cell Mol Life Sci 67:201–216
Arakane Y, Hogenkamp DG, Zhu YC, Kramer KJ, Specht CA, Beeman RW, Kanost MR, Muthukrishnan S (2004) Characterization of two chitin synthase genes of the red flour beetle, Tribolium castaneum, and alternate exon usage in one of the genes during development. Insect Biochem Mol Biol 34:291–304
Bairoch A (1993) The PROSITE dictionary of sites and patterns in proteins, its current status. Nucleic Acids Res 21:3097
Bajda S, Dermauw W, Greenhalgh R, Nauen R, Tirry L, Clark RM, Van Leeuwen T (2015) Transcriptome profiling of a spirodiclofen susceptible and resistant strain of the European red mite Panonychus ulmi using strand-specific RNA-seq. BMC Genom 16:974
Bolognesi R, Arakane Y, Muthukrishnan S, Kramer KJ, Terra WR, Ferreira C (2005) Sequences of cDNAs and expression of genes encoding chitin synthase and chitinase in the midgut of Spodoptera frugiperda. Insect Biochem Mol Biol 35:1249–1259
Boot RG, Renkema GH, Strijland A, van Zonneveld AJ, Aerts JM (1995) Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J Biol Chem 270:26252–26256
Chen J, Zhang D, Yao Q, Zhang J, Dong X, Tian H, Zhang W (2010) Feeding-based RNA interference of a trehalose phosphate synthase gene in the brown planthopper, Nilaparvata lugens. Insect Mol Biol 19:777–786
Chen L, Yang WJ, Cong L, Xu KK, Wang JJ (2013) Molecular cloning, characterization and mRNA expression of a chitin synthase 2 gene from the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Int J Mol Sci 14:17055–17072
Choo YM, Lee KS, Kim BY, Kim DH, Yoon HJ, Sohn HD, Jin BR (2007) A gut-specific chitinase from the mulberry longicorn beetle, Apriona germari (Coleoptera: Cerambycidae): cDNA cloning, gene structure, expression and enzymatic activity. Eur J Entomol 104:173
Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K (1993) Plant chitinases. Plant J 3:31–40
De la Vega H, Specht C, Liu Y, Robbins P (1998) Chitinases are a multi-gene family in Aedes, Anopheles and Drosophila. Insect Mol Biol 7:233–239
Ding TB, Niu JZ, Yang LH, Zhang K, Dou W, Wang JJ (2013) Transcription profiling of two cytochrome P450 genes potentially involved in acaricide metabolism in citrus red mite Panonychus citri. Pestic Biochem Physiol 106:28–37
Dyrløv Bendtsen J, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–795
Eichner C, Harasimczuk E, Nilsen F, Grotmol S, Dalvin S (2015) Molecular characterisation and functional analysis of LsChi2, a chitinase found in the salmon louse (Lepeophtheirus salmonis salmonis, Krøyer 1838). Exp Parasitol 151:39–48
Farlow JE (1976) Dimilin [1-(4-chlorophenyl)-3-(2, 6-difluorobenzoyl)-urea] on the aquatic fauna of a Louisiana coastal marsh. Ph. D. thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA
Funkhouser JD, Aronson NN (2007) Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol 7:96
Hansen JE, Lund O, Rapacki K, Brunak S (1997) O-GLYCBASE version 2.0: a revised database of O-glycosylated proteins. Nucleic Acids Res 25:278–282
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19
Hu J, Wang C, Wang J, You Y, Chen F (2010) Monitoring of resistance to spirodiclofen and five other acaricides in Panonychus citri collected from Chinese citrus orchards. Pest Manage Sci 66:1025–1030
Khajuria C, Buschman LL, Chen MS, Muthukrishnan S, Zhu KY (2010) A gut-specific chitinase gene essential for regulation of chitin content of peritrophic matrix and growth of Ostrinia nubilalis larvae. Insect Biochem Mol Biol 40:621–629
Khila A, Grbić M (2007) Gene silencing in the spider mite Tetranychus urticae: dsRNA and siRNA parental silencing of the Distal-less gene. Dev Genes Evol 217:241–251
Kramer KJ, Muthukrishnan S (2009) Chitin metabolism in insects. Insect development: morphogenesis, molting and metamorphosis/edited by Lawrence I. Gilbert 4:111–144
Kramer KJ, Corpuz L, Choi HK, Muthukrishnan S (1993) Sequence of a cDNA and expression of the gene encoding epidermal and gut chitinases of Manduca Sexta. Insect Biochem Mol Biol 23:691–701
Kramer KJ, Hopkins TL, Schaefer J (1995) Applications of solids NMR to the analysis of insect sclerotized structures. Insect Biochem Mol Biol 25:1067–1080
Kwon DH, Park JH, Lee SH (2013) Screening of lethal genes for feeding RNAi by leaf disc-mediated systematic delivery of dsRNA in Tetranychus urticae. Pestic Biochem Physiol 105:69–75
Leah R, Tommerup H, Svendsen I, Mundy J (1991) Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J Biol Chem 266:1564–1573
Lehane M (1997) Peritrophic matrix structure and function. Annu Rev Entomol 42:525–550
Lehmann PT, White L (1975) Chitin assay used to demonstrate renal localization and cortisone-enhanced growth of Aspergillus fumigatus mycelium in mice. Infect Immun 12:987–992
Li DQ, Zhang JQ, Wang Y, Liu XJ, Ma EB, Suna Y, Li S, Zhu KY, Zhang JZ (2015) Two chitinase 5 genes from Locusta migratoria: molecular characteristics and functional differentiation. Insect Biochem Mol Biol 58:46–55
Liu B, Jiang G, Zhang Y, Li J, Li X, Yue J, Chen F, Liu H, Li H, Zhu S, Wang J, Ran C (2011) Analysis of transcriptome differences between resistant and susceptible strains of the citrus red mite Panonychus citri (Acari: Tetranychidae). PLoS ONE 6:e28516
Mayer R, Chen A, DeLoach J (1981) Chitin synthesis inhibiting insect growth regulators do not inhibit chitin synthase. Experientia 37:337–338
Meola SM, Mayer RT (1980) Inhibition of cellular proliferation of imaginal epidermal cells by diflubenzuron in pupae of the stable fly. Science 207:985–987
Merzendorfer H (2006) Insect chitin synthases: a review. J Comp Physiol Biochem Syst Environ Physiol 176:1–15
Merzendorfer H, Zimoch L (2003) Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol 206:4393–4412
Michel AP, Mian MR, Davila-Olivas NH, Cañas LA (2010) Detached leaf and whole plant assays for soybean aphid resistance: differential responses among resistance sources and biotypes. J Econ Entomol 103:949–957
Migeon A, Dorkeld F (2012) Spider mites web. http://www.montpellier.inra.fr/CBGP/spmweb
Mulder R, Gijswijt MJ (1973) The laboratory evaluation of two promising new insecticides which interfere with cuticle deposition. Pestic Sci 4:737–745
Muzzarelli R, Marks EP (1986) Chitin synthesis inhibitors: effects on insects and on nontarget organisms. Crit Rev Environ Sci Technol 16:141–146
Nakabachi A, Shigenobu S, Miyagishima S (2010) Chitinase-like proteins encoded in the genome of the pea aphid, Acyrthosiphon pisum. Insect Mol Biol 19:175–185
Niu JZ, Liu GY, Dou W, Wang JJ (2011) Susceptibility and activity of glutathione S-transferases in nine field populations of Panonychus citri (Acari: Tetranychidae) to pyridaben and azocyclotin. Fla Entomol 94:321–329
Niu JZ, Dou W, Ding TB, Shen GM, Zhang K, Smagghe G, Wang JJ (2012a) Transcriptome analysis of the citrus red mite, Panonychus citri, and its gene expression by exposure to insecticide/acaricide. Insect Mol Biol 21:422–436
Niu JZ, Dou W, Ding TB, Yang LH, Shen GM, Wang JJ (2012b) Evaluation of suitable reference genes for quantitative RT-PCR during development and abiotic stress in Panonychus citri (McGregor) (Acari: Tetranychidae). Mol Biol Rep 39:5841–5849
Pan Y, Lü P, Wang Y, Yin L, Ma H, Ma G, Chen K, He Y (2012) In silico identification of novel chitinase-like proteins in the silkworm, Bombyx mori, genome. J Insect Sci 12:150
Peters W (1992) Peritrophic membranes. Zoophysiology, vol 30. Springer, Berlin
Pitino M, Coleman AD, Maffei ME, Ridout CJ, Hogenhout SA (2011) Silencing of aphid genes by dsRNA feeding from plants. PLoS ONE 6:e25709
Suzuki M, Fujimoto W, Goto M, Morimatsu M, Syuto B, Iwanaga T (2002) Cellular expression of gut chitinase mRNA in the gastrointestinal tract of mice and chickens. J Histochem Cytochem 50:1081–1089
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Upadhyay SK, Chandrashekar K, Thakur N, Verma PC, Borgio JF, Singh PK, Tuli R (2011) RNA interference for the control of whiteflies (Bemisia tabaci) by oral route. J. Biosci (Bangalore) 36:153–161
Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem Mol Biol 40:563–572
Xi Y, Pan P, Ye Y, Yu B, Xu H, Zhang C (2015) Chitinase-like gene family in the brown planthopper, Nilaparvata lugens. Insect Mol Biol 24:29–40
Xia WK, Ding TB, Niu JZ, Liao CY, Zhong R, Yang WJ, Liu B, Dou W, Wang JJ (2014) Exposure to diflubenzuron results in an up-regulation of a chitin synthase 1 gene in citrus red mite, Panonychus citri (Acari: Tetranychidae). Int J Mol Sci 15:3711–3728
Xu J, Deng X (2008) Current status of citrus industry in China. Citrus Industry in China. China Agriculture Press, Beijing, pp 18–24
Yang WJ, Xu KK, Zhang RY, Dou W, Wang JJ (2013) Transcriptional regulation of a chitinase gene by 20-hydroxyecdysone and starvation in the oriental fruit fly, Bactrocera dorsalis. Int J Mol Sci 14:20048–20063
Zhang JZ, Zhu KY (2006) Characterization of a chitin synthase cDNA and its increased mRNA level associated with decreased chitin synthesis in Anopheles quadrimaculatus exposed to diflubenzuron. Insect Biochem Mol Biol 36:712–725
Zhang JZ, Zhang X, Arakane Y, Muthukrishnan S, Kramer KJ, Ma EB, Zhu KY (2011a) Identification and characterization of a novel chitinase-like gene cluster (AgCht5) possibly derived from tandem duplications in the African malaria mosquito, Anopheles gambiae. Insect Biochem Mol Biol 41:521–528
Zhang JZ, Zhang X, Arakane Y, Muthukrishnan S, Kramer KJ, Ma EB, Zhu KY (2011b) Comparative genomic analysis of chitinase and chitinase-like genes in the african malaria mosquito (Anopheles gambiae). PLoS ONE 6:e19899
Zhang D, Chen J, Yao Q, Pan Z, Chen J, Zhang W (2012) Functional analysis of two chitinase genes during the pupation and eclosion stages of the beet armyworm Spodoptera exigua by RNA interference. Arch Insect Biochem Physiol 79:220–234
Zhang K, Niu JZ, Ding TB, Dou W, Wang JJ (2013) Molecular characterization of two carboxylesterase genes of the citrus red mite, Panonychus citri (Acari: Tetranychidae). Arch Insect Biochem Physiol 82:213–226
Zhu QS, Arakane Y, Banerjee D, Beeman RW, Kramer KJ, Muthukrishnan S (2008a) Domain organization and phylogenetic analysis of the chitinase-like family of proteins in three species of insects. Insect Biochem Mol Biol 38:452–466
Zhu QS, Arakane Y, Beeman RW, Kramer KJ, Muthukrishnan S (2008b) Functional specialization among insect chitinase family genes revealed by RNA interference. Proc Natl Acad Sci USA 105:6650–6655
Acknowledgments
This research was supported in part by Special Fund for Agro-Scientific Research in the Public Interest (201203038), the Fundamental Research Funds for the Central Universities (XDJK2013A017), and the earmarked fund for the Modern Agro-industry (Citrus) Technology Research System of China to Jin-Jun Wang. We also thank Hao Hu and Gang Li for their technical assistance in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, WK., Shen, XM., Ding, TB. et al. Functional analysis of a chitinase gene during the larval-nymph transition in Panonychus citri by RNA interference. Exp Appl Acarol 70, 1–15 (2016). https://doi.org/10.1007/s10493-016-0063-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-016-0063-0