Abstract
Multi-directional interactions occur among plant hosts, Brevipalpus mites and the plant viruses they transmit. Such interactions should be considered when evaluating the severity of a disease such as citrus leprosis. The current understanding of Brevipalpus-transmitted viruses relies on the capability of the vector to transmit the disease, the persistence of the virus in the host plant and the ability of the disease to spread. Previously, we discussed the Citrus leprosis virus (CiLV) and its importance and spread over the past decade into new areas of South and Central America, most recently into southern Mexico and Belize. Here, we address key questions to better understand the biology of the mite vector, fitness costs, and the peculiarities of Brevipalpus mite reproduction, virus survival, transmissibility and spread, and the expansion of the host plant range of Brevipalpus species vectoring the disease.
Similar content being viewed by others
Biological peculiarities of Brevipalpus mite vectors
Previous papers have reviewed the basic biology of several Brevipalpus species on citrus, tea and other plants (Oomen 1982; Haramoto 1969, Chiavegato 1986, Childers et al. 2003a, b). However, new information has come to light that species that have been previously identified as Brevipalpus phoenicis (Geijkes) are actually a complex of morphologically similar species (Beard et al. 2012) and biotypes which co-infest various plant species (Rodrigues et al. 2004, 2008, Kitajima et al. 2010). This requires a closer look at their biology to determine the relationship of the species within the complex that vector diseases with the invasive viruses, such as citrus leprosis and related plant viruses.
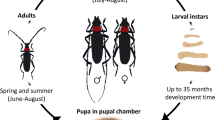
Weeks et al. (2001) found that one of the studied B. phoenicis populations was predominantly thelytokous, in association with the occurrence of feminizing bacteria in the genus Cardinium. However, in mite populations on citrus from the Tocantins State in Brazil (Domingues and Rodrigues 1999) and Florida, males have been observed guarding the immature female teleiochrysalis stage followed by mating, once the adult female emerges (Fig. 1). This same behavior had been reported in the Tetranychoidea superfamily (Collins et al. 1993). Weeks et al. (2001) found that females of B. phoenicis treated with antibiotics that eliminated feminizing bacteria produced higher numbers of males. It does not seem conceivable that males would expend this much output of energy in elaborate female guarding strategies if they were not sexually functional.
Paedogenesis in Brevipalpus mites was first reported by Baker (1972). Occasional events of sexual maturity of immatures were observed in some B. phoenicis colonies that were maintained in the laboratory (Fig. 2). There is no conclusive explanation for the occurrence of this phenomenon or its potential association with sex-alterating symbiont bacteria. Kennedy (1995) suggested that ‘phase variation’ (morphological, behavioral and physiological variations observed within species resulting in many cases of density effects during developmental stage), is another adaptive character associated with the B. phoenicis species complex; this phenomenon may explain the shortening of the developmental time of the mite under situations of high population density.
Paedogenesis occurring in a nymphal stage of Brevipalpus phoenicis. This was first reported by Baker (1972). Some B. phoenicis colonies maintained in the laboratory exhibited occasional events of paedogenesis
Expansion of Brevipalpus transmitted viruses (BTVs)
Brevipalpus transmitted viruses (BTVs) are New World plant pathogens with one known exception, the Orchid fleck virus that is reported worldwide (Kondo et al. 2003). Citrus leprosis-like symptoms were reported to occur in South Africa, Philippines, China, India, Japan, Java and Sri Lanka (Fawcett and Lee 1926, Fawcett 1936), but the causal agent and its vector were not confirmed.
Over the past decade, the spread and economic importance of Citrus leprosis virus (CiLV) into new areas of South and Central America (including Belize) and most recently into southern Mexico has been alarming (Table 1). The spread of this disease across such a large area could be associated with the great increase in the number of hectares of citrus under production in the region (host density) as well as the movement of infected plants and mites from areas where the disease has established. The commonly known species of Brevipalpus mites that infest citrus in tropical and subtropical areas in the Western Hemisphere have a host range that includes hundreds of plant species. Concerns exist about the presence of host plants that are cryptic, asymptomatic, reservoir hosts that can sustain or magnify citrus leprosis-like viruses.
In Brazil, evidence that citrus leprosis was caused by a virus was presented by Kitajima et al. (1972). They identified the occurrence of nuclear (CiLV-N) virus particles associated with sweet orange leaf lesions that were collected from the field. The form of citrus leprosis that occurred in Florida (USA) prior to the 1960s was reported to be the nuclear type of citrus leprosis (Kitajima et al. 2011). Today, CiLV-N is rarely found in commercial citrus orchards in Brazil or elsewhere. The prevalent form of citrus leprosis that is spreading throughout Central America and Mexico is the cytoplasmic (CiLV-C) type (Fig. 3). Key questions remain to be answered: Which virus type was predominant in the past? What led to the near disappearance of the nuclear type and emergence of the cytoplasmic type of citrus leprosis? What factors could be associated with these changes? Where did citrus leprosis and related viruses originate? Are the two virus types from similar origins? Citrus, coffee and many other host plants that are widely grown in Central and South America are not originally from the Western Hemisphere. Are those viruses present within non-symptomatic host plants that serve as reservoirs of infection? Are the many BTVs reported by Kitajima et al. (2010) capable of being transmitted to other plants? Can the mite vectors switch host plants easily (Rodrigues et al. 2005, Nunes et al. 2012)? What role(s) do cryptic species of Brevipalpus that are co-habiting one or more of the hundreds of reported plant hosts play in virus transmission (Childers et al. 2003b, Childers and Rodrigues 2011). In Hawaii, Melzer et al. (2012) reported a leprosis-like disease infecting Citrus volkameriana Tan & Pasq and Hibiscus sp. It could be hypothesized that potential new variations of the viruses emerge because they are constantly evolving and interacting with new or more efficient Brevipalpus vector biotypes.
Symptoms of both the cytoplasmatic form of citrus leprosis (left, CiLV-C) and the nuclear form (right, CiLV-N) in sweet orange leaves. Symptoms of the N type of citrus leprosis were usually smaller than those of the C type. Also, the C type of citrus leprosis had an intense yellow margin around the chlorotic lesion
It has been assumed that the cytoplasmic form of citrus leprosis is more virulent than the nuclear form. This is a speculative assertion and is not supported by direct experimental evidence, but rather based primarily on the frequency of occurrence of characteristic foliar and fruit lesions of the two types in the field. Citrus leprosis reached Mexico around 2004 and spread rapidly through all the major southern citrus-growing areas. Mexico is known to have a rich fauna of false spider mites including many species of Brevipalpus (Baker and Tuttle 1987, Mesa et al. 2009). Recently, both the cytoplasmic and nuclear types of citrus leprosis were identified in Mexico (G. Otero-Colina, pers. comm., 2012). Is it possible that the introduction of both forms of citrus leprosis into southern Mexico overlapped with the new biotypes of mites vectors? This could result in a different pattern of disease spread. To better understand the movement of the disease, it is important to know whether the viruses and the vector biotypes are spreading together, or whether the virus is spreading by vector biotypes or species already occurring in the new region.
Complex pathogen-vector-host interactions such as citrus leprosis represent a challenge for states, countries and regions to effectively establish quarantine measures and barriers. This is despite growing efforts and collaboration among various research institutions and governmental agencies. Biological field studies of Brevipalpus mite populations are needed that include both taxonomic and molecular identification of mite populations not only on citrus but also on adjacent agricultural crops and associated ornamental and ground cover plants including weeds that may serve as host plants.
New mite vectors, virus-infected plants, or virus-infected Brevipalpus mites could arrive into the USA through various pathways (Childers and Rodrigues 2005). Therefore, a proactive approach would be more effective than relying on conventional interception or quarantine measures (Heather and Hallman 2008). A coordinated effort among US citrus producing states is needed to: (1) identify the Brevipalpus species occurring within and around citrus orchards in each state, and (2) to determine the potential of those Brevipalpus species to transmit one or both forms of CiLV. Determining the identity and biology of the Brevipalpus vector(s) and virus types that are spreading in citrus and alternate host plants through Mexico, Belize and other Central American countries is needed. Also, determining the timing, magnitude and dispersal distances of Brevipalpus vector species would provide invaluable information for more effective management capabilities than currently exist (Childers and Rodrigues 2011). Research is needed to minimize the further spread of this serious citrus disease and to reduce potential economic losses.
Transmission of Citrus leprosis virus and vector fitness
The Brevipalpus mite feeding process is crucial in virus acquisition and transmission success (Fig. 4). Two transmission assays were conducted using progeny obtained from a colony of mites established from a single female egg of B. phoenicis (Rodrigues et al. 2004). The original virus isolate used in this study was the cytoplasmic form of citrus leprosis as reported by Locali et al. (2003). Individual mites from the viruliferous colony were transferred to sweet orange seedlings (one mite/plant) and kept under controlled environmental conditions (25 °C, 12:12 light:dark) for 60 days, after which the seedlings were observed for the development of citrus leprosis symptoms. Individual mite survival and colony establishment exceeded 80 % with transmission rates of 10–11 %, for individual adult female mites (Rodrigues 1995). Previous studies conducted in Brazil by Chagas et al. (1983) and Chiavegato (1995) reported transmission rates of citrus leprosis by Brevipalpus mites to be about 8 %. However, neither the transmitted virus type of citrus leprosis nor the Brevipalpus vector species were identified in those studies.
A clonal mite population of B. phoenicis (GenBank Accession AY320019) was divided and maintained over an 18 month interval to assess feeding on both citrus leprosis-infected and healthy tissues. This was done to verify the influence of the cytoplasmic type of citrus leprosis on Brevipalpus fitness. Fitness—evaluated by egg quality through the frequencies of larval hatching—was not affected by the occurrence of citrus leprosis in the mite colony (Table 2).
Symbionts could influence vector behavior and directly or indirectly affect the ability to acquire or transmit the pathogen during the feeding process. Cardinium bacteria were reported to infect Brevipalpus populations (Weeks et al. 2001) but were shown not to affect the fitness of B. californicus mites associated with the transmission of orchid fleck virus in orchids (Chigira and Miura 2005).
Vector-transmitted parasites react to and induce changes in their environment both in the host and vector, as well as in response to other parasites (Matthews 2011). However, very little is known about the molecular and physiological changes in plant hosts and vectors during the process of infection by BTV’s. Considering the physiological changes in leprosis-infected tissues, Nogueira et al. (1996) reported higher levels of iron among other elements associated with citrus leprosis lesions. This was explained by the higher accumulation of ferritin-like arrays associated with pro-plastids on citrus infected cells (Rodrigues 1995). Freitas-Astua et al. (2007) reported the influence of viruses on the expression of plant genes related to plant energy and metabolism in the earlier stages of infection. This agrees with previous cytopathological observations (Rodrigues 1995). In addition, differential host susceptibility to citrus leprosis among citrus species, hybrids and varieties, as shown with ‘Sabará’ tangor and grapefruit could play important roles in virus persistence (Rodrigues 2006).
Social, environmental and economic impacts of Brevipalpus transmitted diseases
The first author visited El Salvador, Central America, in 2010 and met various growers in a citrus cooperative. The group was asked about their major problems in growing citrus and the common answer was ‘mites’. This appeared to be due to the recent introduction and subsequent damage caused by citrus leprosis. A similar situation happened in previous years in Brazil where excessive citrus fruit losses resulted from inability to manage citrus leprosis (Fig. 5; Table 3). Sweet oranges are an important food staple and a major source of vitamins for rural communities throughout Central and South America. Citrus also provides a ready-cash crop for small growers in these countries that sell their produce in local urban markets.
Production costs to control Brevipalpus mites in sweet orange orchards in the state of São Paulo ranged from 12 to 30 % of total production costs or 29–89 % of total phytosanitary expenses (Rodrigues et al. 2002). This does not include losses and costs of eventual pruning when citrus leprosis occurs on tree limbs or branches. Current control approaches for citrus leprosis include progressive inoculum reduction strategies in citrus orchards through scouting and acaricidal spray applications for the mite vector. Using such an integrated approach, losses caused by the disease can be reduced and efficiency of vector control can be enhanced with natural enemies (Fig. 6). Table 3 summarizes yields from various sweet orange varieties (Pera Rio, Valencia, Natal, Hamlin, Seleta, Barão, Lima and Bahia) and Palestine Lime (Citrus limettiodes Tan., asymptomatic to CiLV) that either received or did not receive acaricide sprays. The results were highly variable and were influenced by the orange variety, cost of the control package adopted and potential return to the grower (Dragone et al. 2003). Palestine sweet lime was shown to be immune to CiLV-C and was the only variety that was not negatively affected by the vector-citrus leprosis combination. All sweet orange varieties were severely affected by citrus leprosis in different degrees. In addition to citrus leprosis lowering yields (kg/ha), the remaining fruits on the tree had leprosis symptoms ranging from 57 to 100 % on infested trees compared with 6–47 % on plants that received acaricide sprays. Considering percentage of fruits showing leprosis symptoms, sweet orange varieties Natal, Pera and Seleta were more susceptible, followed by Hamlin, Baía, Valencia, Barão, Lima and Lima Verde. The varieties also showed differential responses to the acaricide treatments.
Microbial pathogens are major natural enemies suppressing Brevipalpus populations. Metarhizium is one of the fungi reported to infect B. phoenicis as shown in (a) (Magalhães et al. 2005). SEM (b) shows fungal hyphae (arrow) growing between the opisthosomal plates
Other hosts plants with BTV’s including coffee, passion fruit and many ornamental plants have been reported by Kitajima et al. (2010) that included new areas of citrus introductions into both agricultural and natural environments (Rodrigues et al. 2008) and urban settings (Miranda et al. 2007). In many instances the initial spread of citrus leprosis goes unnoticed while both movement of people and goods occur.
Conclusions
Brevipalpus mites and viruses that they transmit are a growing phytosanitary threat to the citrus industries of the world. The mites themselves are intriguing organisms in terms of their genetic uniqueness (haploid females showing two non-homologous chromosomes), occurrence of symbionts affecting their sex ratios and the close relationship with numerous cytoplasmic and nuclear types of viruses. To date, these mite-vectored viruses include only non-systemic pathogens that persist under natural conditions and represent an increasing major economic, environmental and social threat to agricultural and ornamental industries.
References
Anonymous (2011) Leprosis outbreak in the citrus industry. http://www.plustvbelize.com/News/NewsDetails/tabid/63/ArticleId/1083/Leprosis-outbreak-in-the-citrus-industry.aspx. Accessed 19 Oct 2011
Araya Gonzales J (2000) Informe sobre la prospeccion de la leprosis de los citricos en la zona fronteriza sur (Costa Rica—Panama). Ministerio de Agricultura y Ganaderia, 5 p
Baker EW (1972) A note on paedogenesis in Brevipalpus sp. (Acari: Tenuipalpidae), the first such record for a mite. Int J Acarol 5:355–356
Baker EW, Tuttle DM (1987). The false spider mites of Mexico (Tenuipalpidae, Acari), U.S. Department of Agriculture Technical Bulletin 1706, 237p
Beard JJ, Ochoa R, Bauchan GR, Trice MD, Redford AJ, Walters TW, Mitter C (2012) Flat mites of the world—part I Raoiella and Brevipalpus. Identification Technology Program, CPHST, PPQ, APHIS, USDA; Fort Collins. http://idtools.org/id/mites/flatmites/. Accessed 14 June 2012)
Bitancourt AA (1940) A leprose dos citros. O Biol 6:39–45
Bitancourt AA (1955) Estudos sobre a leprose dos citrus. Arquivos do Inst Biol de São Paulo 22:161–231
Bitancourt AA, Grillo HVS (1934) A clorose zonada—uma nova doença dos citrus. Arquivos do Inst Biol 5:245–251
Bitancourt AA, Fonseca JP, Autori M (1933) Doenças, pragas e tratamentos. In: Andrade EN (ed) Manual de citricultura. Chácaras e Quintaes pt2, São Paulo 321p
Chagas CM, Rossetti V, Chiavegato LG (1983) Effectiveness of the different life cycle stages of Brevipalpus phoenicis Geijskes in leprosis transmission. In: Proceedings of international organization of citrus virologists, Riverside, California 9, pp 211–214
Chiavegato LG (1986) Biologia do ácaro Brevipalpus phoenicis em citros. Pesqui Agropecu Bras 21:813–816
Chiavegato LG (1995) Avaliação da potencialidade de Brevipalpus phoenicis (Geijskes, 1939) (Acari: Tenuipalpidae) na transmissão da leprose em plantas cítricas. In: Congresso Brasileiro de Entomologia, Caxambu 15, 14
Chigira A, Miura K (2005) Detection of ‘Candidatus Cardinium’ bacteria from the haploid host Brevipalpus californicus (Acari: Tenuipalpidae) and effect on the host. Exp Appl Acarol 37:107–116
Childers CC, Rodrigues JCV (2005) Potential pest mite species collected on ornamental plants from Central America at port of entry to the United States. Fla Entomol 88(4):409–414
Childers CC, Rodrigues JCV (2011) An overview of Brevipalpus mites (Acari: Tenuipalpidae) and the plant viruses they transmit. Zoosymposia 6:168–180
Childers CC, French JV, Rodrigues JCV (2003a) Brevipalpus californicus, B. obovatus, B. phoenicis, and B. lewisi (Acari: Tenuipalpidae): a review of their biology, feeding injury and economic importance. Exp Appl Acarol 30:5–28
Childers CC, Rodrigues JCV, Welbourn WC (2003b) Host plants of Brevipalpus californicus, B. obovatus, and B. phoenicis (Acari: Tenuipalpidae) and their potential involvement in the spread of viral diseases vectored by these mites. Exp Appl Acarol 30:29–105
Collins RD, Margolies DC, Rose S (1993) Guarding behavior and reproductive isolation in 2 tetranychid mite species (Acari, Tetranychidae). Ann Entomol Soc Am 86:111–116
Domingues AD, Rodrigues JCV (1999) Ocorrência de leprose dos citros em Paraíso do Tocantins (TO). Laranja 20(1):35–50
Dominguez FS, Bernal A, Childers CC, Kitajima EW (2001) First report of citrus leprosis on Panama. Plant Dis 85:228
Dragone D, Rodrigues JCV, Neves EM, Nogueira NL (2003) Viabilidade econômica do controle da leprose em variedades de laranjas e lima da Persia. Laranja 24:311–328
Fawcett HS (1936) Citrus diseases and their control, 2nd edn. McGraw-Hill, New York
Fawcett HS, Lee HA (1926) Citrus diseases and their control. McGraw-Hill, New York 582 p
Freitas-Astua J, Bastianel M, Locali-Fabris EC, Novelli VM, Silva-Pinhati AC, Basilio-Palmieri AC, Targon LPN, Machado MA (2007) Differentially expressed stress-related genes in the compatible citrus-Citrus leprosis virus interaction. Genet Mol Biol 30:980–990
Haramoto FH (1969) Biology and control of Brevipalpus phoenicis (Geijskes) (Acarina: Tenuipalpidae). Hawaii Agric Exp Stn Tech Bull 68:1–63
Heather NW, Hallman GJ (2008) Managing risk of pest introduction, establishment and spreading in a changing world. In: Pest management and phytosanitary trade barriers, CABI, Wallingford, UK, pp 27–46
Kennedy JS (1995) Phase variation—a possible adaptive character for the false spider mite, Brevipalpus phoenicis (Geijskes 1939). J Appl Entomol 119:259–261
Kitajima EW, Muller GW, Costa AS, Yuki V (1972) Short rod-like particles associated with citrus leprosis. Virology 50:254–258
Kitajima EW, Rodrigues JCV, Freitas-Astua J (2010) An annotated list of ornamentals naturally found infected by Brevipalpus mite transmitted viruses. Sci Agric 67(3):348–371
Kitajima EW, Chagas CM, Harakava R, Calegario RF, Freitas-Astúa J, Rodrigues JCV, Childers CC (2011). Citrus Leprosis in Florida, USA, appears to have been caused by the Nuclear Type of Citrus Leprosis Virus (CiLV-N). Virus Rev Res 16:1–5
Kondo H, Maeda T, Tamada T (2003) Orchid fleck virus: Brevipalpus californicus mite transmission, biological properties and genome structure. Exp Appl Acarol 30:215–223
Leon GA, Realpe MCE, Garzon PA, Rodriguez JA, Moreno MG, Childers CC, Achor D, Freitas-Astua J, Antonioli-Luizon R, Salaroli RB, Mesa NC, Kitajima EW (2006) Occurrence of citrus leprosis virus in Llanos Orientales, Colombia. Plant Dis 90:692
Locali EC, Freitas-Astua J, Souza AA, Takita MA, Astua-Monge G, Antonioli R, Kitajima EW, Machado MA (2003) Development of a molecular tool for the diagnosis of leprosis, a major threat to citrus production in the Americas. Plant Dis 87:1317–1321
Magalhães BP, Rodrigues JCV, Boucias DG, Childers CC (2005) Pathogenicity of Metarhizium anisopliae var. acridum to the flat mite Brevipalpus phoenicis (Acari: Tenuipalpidae). Fla Entomol 88(2):195–198
Matthews KR (2011) Controlling and coordinating development in vector-transmitted parasites. Science 331:1149–1153
Mejia L, Paniagua A, Cruz N, Porras M, Palmieri M (2002) Citrus leprosis, disease that endangers plantations in Guatemala. In: Proceedings of 42nd annual meeting American Phytopathological Society, Caribbean Division, Antigua, Guatemala, 17–19 June (Abstract)
Melzer MJ, Sether DM, Borth WB, Hu JS (2012) Characterization of a virus infecting Citrus volkameriana with citrus leprosis-like symptoms. Phytopathology 102:122–127
Mesa NC, Ochoa R, Welbourn WC, Evans GA, De Moraes GJ (2009) A catalog of the Tenuipalpidae (Acari) of the world with a key to genera. Zootaxa 2098: 1-185. Magnolia Press, Auckland
Miranda LC, Navia D, Rodrigues JCV (2007) Brevipalpus Donnadieu mites (Prostigmata: Tenuipalpidae) associated with ornamental plants in Distrito Federal, Brazil. Neotropical Entomol 36(4):587–592
Nogueira NL, Rodrigues JCV, Cabral CP, Prates HS (1996) The influence of citrus leprosis disease on the mineral composition of Citrus sinensis leaves. Sci Agric 53:354–355
Nunes MA, Oliveira CAL, Oliveira ML, Kitajima EW, Hilf ME, Gottwald T, Freitas-Astua J (2012) Transmission of Citrus leprosis virus C by Brevipalpus phoenicis (Geijskes) to alternative host plants found in citrus orchards. Plant Dis 96:968–972
Oomen PA (1982) Studies on population dynamics of the scarlet mite, Brevipalpus phoenicis, a pest of tea in Indonesia. Med. Landbouwhogeschool 82-1 Wageningen
Rodrigues JCV (1995) Leprose dos citros: cito-patologia, transmissibilidade e relação com o vetor Brevipalpus phoenicis Geijskes (Acari: Tenuipalpidae). MSc Thesis, University of São Paulo, Piracicaba, SP, 79 pp
Rodrigues JCV (2006) ‘Is ‘Sabará’ sweet orange immune or resistant to citrus leprosis?’: addressing Bitancourt’s 1938 question. Laranja 27(1):31–39
Rodrigues JCV, Prates HS, Nogueira NL, Freitas DS, Rossi ML (1995) Leprose dos citros: relação vetor × patógeno × planta. Laranja 16(2):97–106
Rodrigues JCV, Ueta FZ, Muraro RP (2002) Opções e custos comparativos para um programa de redução do inóculo da leprose dos citros. Laranja 23:333–344
Rodrigues JCV, Childers CC, Gallo-Meagher M, Ochoa R, Adams BJ (2004) Mitochondrial DNA and RAPD polymorphisms in the haploid mite Brevipalpus phoenicis (Acari: Tenuipalpidae). Exp Appl Acarol 34:274–290
Rodrigues JCV, Locali EC, Freitas-Astua J, Kitajima EW (2005) Transmissibility of citrus leprosis virus by Brevipalpus phoenicis to Solanum violaefolium. Plant Dis 89:911
Rodrigues JCV, Zuniga JA, Childers CC (2007) Occurrence and distribution of citrus leprosis virus in Honduras. Plant Pathol 56:344
Rodrigues JCV, Kriger LA, Salaroli RB, Kitajima EW (2008) Brevipalpus-associated viruses in the Central Amazon Basin. Tropical Plant Pathol 33(1):12–19
SAGARPA (2009) Acciones contra la leprosis de los citricos. Available from http//www.senasica.gob.mx/includes/asp/download.asp?iddocumento=20932&idurl=37611. Accessed Aug 2011
Spegazzini C (1920) Sôbre algunas enfermedades y hongos que afectan las plantas de ‘agrios’ en el Paraguay. Ann Soc Cient Argent 90:155–188
Teixeira CDA, Avile DP, Rodrigues VGS, Ferreira MG (1993) Levantamento da ocorrência da leprose dos citros causada por Brevipalpus phoenicis (Acari:Tenuipalpidae) em Rondônia. In: Congresso Brasileiro de Entomologia 14, Piracicaba, SP, p 717
Weeks A, Marec F, Breeuwer JAJ (2001) A mite species that consists entirely of haploid females. Science 292:2479–2482
Acknowledgments
We would like to thank Dr. Neusa L. Nogueira and Monica Lanzoni (Univ. São Paulo) and Diann S. Achor (Univ Florida) for their initial support and advice using electron microscopy analysis. Appreciation is extended to Dr. Elliot Kitajima (Univ. São Paulo) for fruitful discussions on CiLV-Brevipalpus interactions. The following funding agencies supported parts of the research presented in this paper: Fundecitrus, CNPq, CAPES (Brazil), USDA (CA Awards), and the California Citrus Research Board.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Rodrigues, J.C.V., Childers, C.C. Brevipalpus mites (Acari: Tenuipalpidae): vectors of invasive, non-systemic cytoplasmic and nuclear viruses in plants. Exp Appl Acarol 59, 165–175 (2013). https://doi.org/10.1007/s10493-012-9632-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-012-9632-z