Abstract
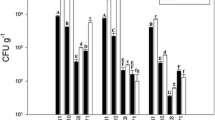
Little is known about the composition, diversity, and geographical distribution of bacterial communities associated with medicinal plants in arid lands. To address this, a collection of 116 endophytic bacteria were isolated from wild populations of the herb Glycyrrhiza uralensis Fisch (licorice) in Xinyuan, Gongliu, and Tekesi of Xinjiang Province, China, and identified based on their 16S rRNA gene sequences. The endophytes were highly diverse, including 20 genera and 35 species. The number of distinct bacterial genera obtained from root tissues was higher (n = 14) compared to stem (n = 9) and leaf (n = 6) tissue. Geographically, the diversity of culturable endophytic genera was higher at the Tekesi (n = 14) and Xinyuan (n = 12) sites than the Gongliu site (n = 4), reflecting the extremely low organic carbon content, high salinity, and low nutrient status of Gongliu soils. The endophytic bacteria exhibited a number of plant growth-promoting activities ex situ, including diazotrophy, phosphate and potassium solubilization, siderophore production, auxin synthesis, and production of hydrolytic enzymes. Twelve endophytes were selected based on their ex situ plant growth-promoting activities for growth chamber assays to test for their ability to promote growth of G. uralensis F. and Triticum aestivum (wheat) plants. Several strains belonging to the genera Bacillus (n = 6) and Achromobacter (n = 1) stimulated total biomass production in both G. uralensis and T. aestivum under low-nutrient conditions. This work is the first report on the isolation and characterization of endophytes associated with G. uralensis F. in arid lands. The results demonstrate the broad diversity of endophytes associated with wild licorice and suggest that some Bacillus strains may be promising candidates for biofertilizers to promote enhanced survival and growth of licorice and other valuable crops in arid environments.




Similar content being viewed by others
References
Ahmed E, Holmström SJ (2014) Siderophores in environmental research: roles and applications. Microb Biotechnol 7:196–208
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12:39–45
Bajguz A (2007) Metabolism of brassinosteroids in plants. Plant Physiol Biochem 45:95–107
Basak B, Biswas D (2010) Co-inoculation of potassium solubilizing and nitrogen fixing bacteria on solubilization of waste mica and their effect on growth promotion and nutrient acquisition by a forage crop. Biol Fertil Soils 46:641–648
Bøckman OC (1997) Fertilizers and biological nitrogen fixation as sources of plant nutrients: perspectives for future agriculture. Plant Soil 194:11–14
Boor KJ (2006) Bacterial stress responses: what doesn’t kill them can make them stronger. PLoS Biol 4:e23
Brown MRW, Foster JS (1970) A simple diagnostic milk medium for Pseudomonas aeruginosa. J Clin Pathol 23:172–177
Chand S, Mishra P (2003) Research and application of microbial enzymes—India’s contribution. Biotechnol India II:95–124
Chelius MK, Triplett EW (2000) Immunolocalization of dinitrogenase reductase produced by Klebsiella pneumoniae in association with zea mays L. Appl Environ Microbiol 66:783–787
Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK et al (2007) EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 57:2259–2261
Cushnie TT, Cushnie B, Lamb AJ (2014) Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents 44:377–386
Daffonchio D, Hirt H, Berg G (2015) Plant–microbe interactions and water management in arid and saline soils. In: Lugtenberg B (ed) Principles of plant–microbe interactions. Springer, Cham
Das G, Park S, Baek KH (2017) Diversity of endophytic bacteria in a fern species Dryopteris uniformis (Makino) Makino and evaluation of their antibacterial potential against five foodborne pathogenic bacteria. Foodborne Pathog Dis 14:260–268
Donate-Correa J, León-Barrios M, Pérez-Galdona R (2005) Screening for plant growth-promoting rhizobacteria in Chamaecytisus proliferus (tagasaste), a forage tree-shrub legume endemic to the Canary Islands. Plant Soil 266:261–272
Dong Y, Chelius MK, Brisse S, Kozyrovska N, Kovtunovych G, Podschun R et al (2003) Comparisons between two Klebsiella: the plant endophyte K. pneumoniae 342 and a clinical isolate, K. pneumoniae MGH78578. Symbiosis 35:247–259
Egamberdieva D, Wirth S, Behrendt U, Ahmad P, Berg G (2017) Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front Microbiol 8:199
Egamberdiyeva D (2007) The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol 36:184–189
Gasser I, Cardinale M, Müller H, Heller S, Eberl L, Lindenkamp N et al (2011) Analysis of the endophytic lifestyle and plant growth promotion of Burkholderia terricola ZR2-12. Plant Soil 347:125–136
Giassi V, Kiritani C, Kupper KC (2016) Bacteria as growth-promoting agents for citrus rootstocks. Microbiol Res 190:46–54
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192
Halo BA, Khan AL, Waqas M, Al-Harrasi A, Hussain J, Ali L et al (2015) Endophytic bacteria (Sphingomonas sp. LK11) and gibberellin can improve Solanum lycopersicum growth and oxidative stress under salinity. J Plant Interact 10:117–125
Hameeda B, Harini G, Rupela O, Wani S, Reddy G (2008) Growth promotion of maize by phosphate-solubilizing bacteria isolated from composts and macrofauna. Microbiol Res 163:234–242
Islam F, Yasmeen T, Arif MS, Ali S, Ali B, Hameed S et al (2016) Plant growth promoting bacteria confer salt tolerance in Vigna radiata. Plant Growth Regul 1:23–36
Jin H, Yang XY, Yan ZQ, Liu Q, Li XZ, Chen JX et al (2014) Characterization of rhizosphere and endophytic bacterial communities from leaves, stems and roots of medicinal Stellera chamaejasme L. Syst Appl Microbiol 37:376–385
Kandel SL, Firrincieli A, Joubert PM, Okubara PA, Leston ND, McGeorge KM et al (2017) An in vitro study of bio-control and plant growth promotion potential of Salicaceae endophytes. Front Microbiol 8:386
Kaplan D, Maymon M, Agapakis CM, Lee A, Wang A, Prigge BA et al (2013) A survey of the microbial community in the rhizosphere of two dominant shrubs of the Negev Desert highlands, Zygophyllum dumosum (Zygophyllaceae) and Atriplex halimus (Amaranthaceae), using cultivation-dependent and cultivation-independent methods. Am J Bot 100:1713–1725
Khan AL, Waqas M, Kang SM, Al-Harrasi A, Hussain J, Al-Rawahi A et al (2014) Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J Microbiol 52:689–695
Knudsen D, Peterson GA, Pratt PF (1982) Lithium, sodium and potassium. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis part 2: chemical and microbiologicaI properties, 2nd edn. American Society of Agronomy, Soil Science Society of America, Madison, pp 225–246
Kumar P, Khare S, Dubey R (2012) Diversity of Bacilli from disease suppressive soil and their role in plant growth promotion and yield enhancement. N Y Sci J 5:90–111
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Lemanceau P, Bauer P, Kraemer S, Briat JF (2009) Iron dynamics in the rhizosphere as a case study for analyzing interactions between soils, plants and microbes. Plant Soil 321:513–535
Leveau JH, Lindow SE (2005) Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl Environ Microbiol 71:2365–2371
Lewis G, Schrire B, Mackinder B, Lock M (2005) Legumes of the world. Royal Botanic Gardens, Kew, London
Li L, Sinkko H, Montonen L, Wei GH, Lindstrom K, Rasanen LA (2012) Biogeography of symbiotic and other endophytic bacteria isolated from medicinal Glycyrrhiza species in China. FEMS Microbiol Ecol 79:46–68
Lin L, Li Z, Hu C, Zhang X, Chang S, Yang L et al (2012) Plant growth-promoting nitrogen-fixing enterobacteria are in association with sugarcane plants growing in Guangxi, China. Microbes Environ 27:391–398
Liu YH, Guo JW, Salam N, Li L, Zhang YG, Han J et al (2016) Culturable endophytic bacteria associated with medicinal plant Ferula songorica: molecular phylogeny, distribution and screening for industrially important traits. 3. Biotech 6:209
Liu Y, Guo J, Li L, Asem MD, Zhang Y, Mohamad OA et al (2017) Endophytic bacteria associated with endangered plant Ferula sinkiangensis KM Shen in an arid land: diversity and plant growth-promoting traits. J Arid Land 9:432–445
Ma B, Lv X, Warren A, Gong J (2013) Shifts in diversity and community structure of endophytic bacteria and archaea across root, stem and leaf tissues in the common reed, Phragmites australis, along a salinity gradient in a marine tidal wetland of northern China. Antonie Van Leeuwenhoek 104:759–768
Malfanova N, Kamilova F, Validov S, Shcherbakov A, Chebotar V, Tikhonovich I et al (2011) Characterization of Bacillus subtilis HC8, a novel plant-beneficial endophytic strain from giant hogweed. Microbial Biotechnol 4:523–532
Nanjing Institute of Soil Research, CAS (1980) Analysis of soil physicochemical features. Shanghai Science and Technology Press, Shanghai (in Chinese)
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Klute A (ed) Methods of soil analysis, Part 2, 2nd edn. Agron. Monogr. 9. ASA. Madison
Nimaichand S, Devi AM, Li WJ (2016) Direct plant growth-promoting ability of actinobacteria in grain legumes. In: Plant growth promoting actinobacteria. Springer, New york, pp 1–16
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis part 2: chemical and microbiologicaI properties, 2nd edn. Soil Science Society of America. American Society of Agronomy, Madison, pp 403–430
Patil SV, Jayamohan NS, Kumudini BS (2016) Strategic assessment of multiple plant growth promotion traits for shortlisting of fluorescent Pseudomonas spp. and seed priming against ragi blast disease. Plant Growth Regul 80:47–58
Paul D, Sinha SN (2017) Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river Ganga, India. Ann Agrar Sci 15:130–136
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370 (in Russian)
Qin S, Chen HH, Zhao GZ, Li J, Zhu WY, Xu LH et al (2012) Abundant and diverse endophytic actinobacteria associated with medicinal plant Maytenus austroyunnanensis in Xishuangbanna tropical rainforest revealed by culture dependent and culture independent methods. Environ Microbiol Rep 4:522–531
Rao S (1977) Soil microorganisms and plant growth. Oxford and IBH Publishing Co, India
Rastogi G, Sbodio A, Tech JJ, Suslow TV, Coaker GL, Leveau JH (2012) Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J 6:1812–1822
Reinhold-Hurek B, Hurek T (2011) Living inside plants: bacterial endophytes. Curr Opin Plant Biol 14:435–443
Sánchez-López AS, Thijs S, Beckers B, González-Chávez MC, Weyens N, Carrillo-González R et al (2017) Community structure and diversity of endophytic bacteria in seeds of three consecutive generations of Crotalaria pumila growing on metal mine residues. Plant Soil 422:51–66
Sen M, Sen SP (1965) Interspecific transformation in Azotobacter. Microbiology 41:1–6
Sibanda T, Selvarajan R, Tekere M (2017) Synthetic extreme environments: overlooked sources of potential biotechnologically relevant microorganisms. Microbial Biotechnol 10:570–585
Sierra G (1957) A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Van Leeuwenhoek 23:15–22
Strobel G, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Szymańska S, Płociniczak T, Piotrowska-Seget Z, Hrynkiewicz K (2016) Endophytic and rhizosphere bacteria associated with the roots of the halophyte Salicornia europaea L. community structure and metabolic potential. Microbiol Res 192:37–51
Teather RM, Wood PJ (1982) Use of Congo red–polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43:777–780
Trivedi P, Spann T, Wang N (2011) Isolation and characterization of beneficial bacteria associated with citrus roots in florida. Microb Ecol 62:324–336
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Wei L, Shao Y, Wan J, Feng H, Zhu H, Huang H et al (2014) Isolation and characterization of a rhizobacterial antagonist of root-knot nematodes. PLoS ONE 9:e85988
Wemheuer F, Kaiser K, Karlovsky P, Daniel R, Vidal S, Wemheuer B (2017) Bacterial endophyte communities of three agricultural important grass species differ in their response towards management regimes. Sci Rep 7:40914
Wong WT, Tseng CH, Hsu SH, Lur HS, Mo CW, Huang CN et al (2014) Promoting effects of a single Rhodopseudomonas palustris inoculant on plant growth by Brassica rapa chinensis under low fertilizer input. Microbes Environ 29:303–313
Zakry FAA, Shamsuddin ZH, Abdul RK, Zawawi ZZ, Abdul RA (2012) Inoculation of Bacillus sphaericus UPMB-10 to young oil palm and measurement of its uptake of fixed nitrogen using the 15 N isotope dilution technique. Microbes Environ 27:257–262
Zhang Q, Ye M (2009) Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J Chromatog A 1216:1954–1969
Acknowledgements
This work was supported by Xinjiang Uygur Autonomous Region regional coordinated innovation project (Shanghai Cooperation Organization Science and Technology Partnership Program) (No. 2017E01031). Li Li was supported by China Scholarship Council to study in the United States of America (No. 201509655013). Osama A. A. Mohamad was supported by Chinese Academy of Sciences President’s International Fellowship Initiative (No. 2016PB024) and Available Position Talented Young Scientists Program of Ministry of Science and Technology of the People’s Republic of China (No. P-EG-16-01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no indirect or direct conflict of interest.
Ethical approval
This article does not contain any studies related to human participants or animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10482_2018_1062_MOESM1_ESM.tiff
Figure S1. Bacterial species distribution of culturable endophytic isolates from Glycyrrhiza uralensis F based on 16S rRNA gene sequences for all sites. Supplementary material 1 (TIFF 764 kb)
10482_2018_1062_MOESM2_ESM.tiff
Figure S2. Distribution of endophytic isolates and species isolated from Glycyrrhiza uralensis F based on different media. Supplementary material 2 (TIFF 688 kb)
10482_2018_1062_MOESM3_ESM.tiff
Figure S3. Confirmation of plant growth promotion traits by a color change or halo zone on the selective medium (A: Protease; B: Cellulase; C: Lipase; D: Nitrogen; E: Phosphorus; F: Potassium; G: Siderophore; H: IAA). Supplementary material 3 (TIFF 2689 kb)
10482_2018_1062_MOESM4_ESM.tiff
Figure S4. Effect of endophytic isolates on the growth of Glycyrrhiza uralensis F compared with an uninoculated control (CK). Supplementary material 4 (TIFF 4374 kb)
10482_2018_1062_MOESM5_ESM.tiff
Figure S5. Effect of endophytic isolates on the growth of Triticum aestivum compared with an uninoculated control (CK). Supplementary material 5 (TIFF 3684 kb)
Rights and permissions
About this article
Cite this article
Li, L., Mohamad, O.A.A., Ma, J. et al. Synergistic plant–microbe interactions between endophytic bacterial communities and the medicinal plant Glycyrrhiza uralensis F.. Antonie van Leeuwenhoek 111, 1735–1748 (2018). https://doi.org/10.1007/s10482-018-1062-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-018-1062-4