Abstract
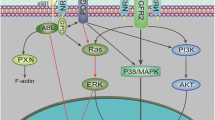
The semaphorins and plexins comprise a family of cysteine-rich proteins implicated in control of nerve growth and development and regulation of the immune response. Our group and others have found that Semaphorin 4D (SEMA4D) and its receptor, Plexin-B1, play an important role in tumor-induced angiogenesis, with some neoplasms producing SEMA4D in a manner analogous to vascular endothelial growth factor (VEGF) in order to attract Plexin-B1-expressing endothelial cells into the tumor for the purpose of promoting growth and vascularity. While anti-VEGF strategies have been the focus of most angiogenesis inhibition research, such treatment can lead to upregulation of pro-angiogenic factors that can compensate for the loss of VEGF, eventually leading to failure of therapy. Here, we demonstrate that SEMA4D cooperates with VEGF to promote angiogenesis in malignancies and can perform the same function in a setting of VEGF blockade. We also show the potential value of inhibiting SEMA4D/Plexin-B1 signaling as a complementary mechanism to anti-VEGF treatment, particularly in VEGF inhibitor–resistant tumors, suggesting that this may represent a novel treatment for some cancers.







Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Change history
10 March 2020
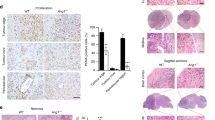
The Editors-in-Chief have retracted this article [1] following an investigation by the University of Maryland. The institution found that in Figures 1B and 1D, the cell lines are different and all published histograms show SEMA4D mRNA level whereas Excel data have two histograms showing SEMA4D expression and two histograms showing VEGF expression. In Figure 2B, the metadata for one image shows different treatment conditions than those reported in the article. The published image labelled VEGF + VEGFR-2 shRNA has a metadata label of S4d-plexinB1 shRNA2. In Figure 2E, statistical significance was shown in the published figure for four comparisons, but upon recalculation, one comparison noted as significant was not. In Figure 6A, the lower left image is labelled VEGF shRNA in the published figure, but the metadata label is S4DshRNA-HN121-20X. In Figure 6C, specifically, within columns 2-4, for each antibody used for immunocytochemistry, the three images have been swapped so that the original images do not match the shRNA labels in the figure (the labels for the two antibodies were correct). In Figure 7D, the first published image is labelled as IgG in the paper, but the metadata show a label of Restore (V+S).tif. The third published image has a label of anti-VEGF IgG, and the metadata show a label of con sh.tif. Due to these errors, the Editors-in-Chief have found that the results are no longer reliable.
10 March 2020
The Editors-in-Chief have retracted this article [1] following an investigation by the University of Maryland. The institution found that in Figures 1B and 1D, the cell lines are different and all published histograms show SEMA4D mRNA level whereas Excel data have two histograms showing SEMA4D expression and two histograms showing VEGF expression. In Figure 2B, the metadata for one image shows different treatment conditions than those reported in the article. The published image labelled ���VEGF + VEGFR-2 shRNA��� has a metadata label of S4d-plexinB1 shRNA2���. In Figure 2E, statistical significance was shown in the published figure for four comparisons, but upon recalculation, one comparison noted as significant was not. In Figure 6A, the lower left image is labelled ���VEGF shRNA��� in the published figure, but the metadata label is ���S4DshRNA-HN121-20X���. In Figure 6C, specifically, within columns 2-4, for each antibody used for immunocytochemistry, the three images have been swapped so that the original images do not match the shRNA labels in the figure (the labels for the two antibodies were correct). In Figure 7D, the first published image is labelled as ���IgG��� in the paper, but the metadata show a label of ���Restore (V+S).tif���. The third published image has a label of ���anti-VEGF IgG���, and the metadata show a label of ���con sh.tif���. Due to these errors, the Editors-in-Chief have found that the results are no longer reliable.
References
Zachary I (2003) Vascular endothelial growth factor and anti-angiogenic peptides as therapeutic and investigational molecules. IDrugs 6:224–231
Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M (1998) Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA 95:9349–9354
Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7:1194–1201
Semenza GL (2001) Regulation of hypoxia-induced angiogenesis: a chaperone escorts VEGF to the dance. J Clin Invest 108:39–40
Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N, Giacca M, Stassen JM, Dewerchin M, Collen D, Carmeliet P (2007) Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131:463–475
Kumanogoh A, Kikutani H (2001) The CD100–CD72 interaction: a novel mechanism of immune regulation. Trends Immunol 22:670–676
Hall KT, Boumsell L, Schultze JL, Boussiotis VA, Dorfman DM, Cardoso AA, Bensussan A, Nadler LM, Freeman GJ (1996) Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc Natl Acad Sci USA 93:11780–11785
Carmeliet P, Tessier-Lavigne M (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436:193–200
Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM (1999) Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99:59–69
Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van NP, Fishman MC, Childs S, Epstein JA, Weinstein BM (2004) Semaphorin–plexin signaling guides patterning of the developing vasculature. Dev Cell 7:117–123
Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M (2002) Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci USA 99:3657–3662
Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS (2004) Class IV semaphorins promote angiogenesis by stimulating rho-initiated pathways through plexin-B. Cancer Res 64:5212–5224
Basile JR, Holmbeck K, Bugge TH, Gutkind JS (2007) MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem 282:6899–6905
Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, Bussolino F, Giordano S (2005) Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood 105:4321–4329
Cardinali M, Pietraszkiewicz H, Ensley JF, Robbins KC (1995) Tyrosine phosphorylation as a marker for aberrantly regulated growth-promoting pathways in cell lines derived from head and neck malignancies. Int J Cancer 61:98–103
Siolas D, Lerner C, Burchard J, Ge W, Linsley PS, Paddison PJ, Hannon GJ, Cleary MA (2005) Synthetic shRNAs as potent RNAi triggers. Nat Biotechnol 23:227–231
Hannon GJ, Conklin DS (2004) RNA interference by short hairpin RNAs expressed in vertebrate cells. Methods Mol Biol 257:255–266
Basile JR, Castilho RM, Williams VP, Gutkind JS (2006) Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. PNAS 103:9017–9022
Paddison PJ, Caudy AA, Sachidanandam R, Hannon GJ (2004) Short hairpin activated gene silencing in mammalian cells. Methods Mol Biol 265:85–100
Paddison PJ, Silva JM, Conklin DS, Schlabach M, Li M, Aruleba S, Balija V, O’Shaughnessy A, Gnoj L, Scobie K, Chang K, Westbrook T, Cleary M, Sachidanandam R, McCombie WR, Elledge SJ, Hannon GJ (2004) A resource for large-scale RNA-interference-based screens in mammals. Nature 428:427–431
Naldini L, Blomer U, Gage FH, Trono D, Verma IM (1996) Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA 93:11382–11388
Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D (1996) In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263–267
Stone D, Phaneuf M, Sivamurthy N, LoGerfo FW, Quist WC (2002) A biologically active VEGF construct in vitro: implications for bioengineering-improved prosthetic vascular grafts. J Biomed Mater Res 59:160–165
Wang F, Yamauchi M, Muramatsu M, Osawa T, Tsuchida R, Shibuya M (2011) RACK1 regulates VEGF/Flt1-mediated cell migration via activation of a PI3 K/Akt pathway. J Biol Chem 286:9097–9106
Carretero-Ortega J, Walsh CT, Hernandez-Garcia R, Reyes-Cruz G, Brown JH, Vazquez-Prado J (2010) Phosphatidylinositol 3,4,5-triphosphate-dependent Rac exchanger 1 (P-Rex-1), a guanine nucleotide exchange factor for Rac, mediates angiogenic responses to stromal cell-derived factor-1/chemokine stromal cell derived factor-1 (SDF-1/CXCL-12) linked to Rac activation, endothelial cell migration, and in vitro angiogenesis. Mol Pharmacol 77:435–442
Mirzapoiazova T, Kolosova I, Usatyuk PV, Natarajan V, Verin AD (2006) Diverse effects of vascular endothelial growth factor on human pulmonary endothelial barrier and migration. Am J Physiol Lung Cell Mol Physiol 291:L718–L724
Guedez L, Rivera AM, Salloum R, Miller ML, Diegmueller JJ, Bungay PM, Stetler-Stevenson WG (2003) Quantitative assessment of angiogenic responses by the directed in vivo angiogenesis assay. Am J Pathol 162:1431–1439
Sahagun G, Moore SA, Fabry Z, Schelper RL, Hart MN (1989) Purification of murine endothelial cell cultures by flow cytometry using fluorescein-labeled griffonia simplicifolia agglutinin. Am J Pathol 134:1227–1232
Laitinen L (1987) Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. Histochem J 19:225–234
Amornphimoltham P, Sriuranpong V, Patel V, Benavides F, Conti CJ, Sauk J, Sausville EA, Molinolo AA, Gutkind JS (2004) Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: a potential target for UCN-01. Clin Cancer Res 10:4029–4037
Sun Q, Zhou H, Binmadi NO, Basile JR (2009) Hypoxia-inducible factor-1-mediated regulation of semaphorin 4D affects tumor growth and vascularity. J Biol Chem 284:32066–32074
Elhabazi A, Delaire S, Bensussan A, Boumsell L, Bismuth G (2001) Biological activity of soluble CD100. I. The extracellular region of CD100 is released from the surface of T lymphocytes by regulated proteolysis. J Immunol 166:4341–4347
Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, Tamagnone L, Wagner DD, Milla ME, Brass LF (2007) Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci USA 104:1621–1626
Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le Couter J, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ (2007) Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 11:53–67
Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91:1071–1121
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307
Ferrara N (2010) Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev 21:21–26
Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL (2002) MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell 109:887–900
Siebold C, Berrow N, Walter TS, Harlos K, Owens RJ, Stuart DI, Terman JR, Kolodkin AL, Pasterkamp RJ, Jones EY (2005) High-resolution structure of the catalytic region of MICAL (molecule interacting with CasL), a multidomain flavoenzyme-signaling molecule. Proc Natl Acad Sci USA 102:16836–16841
Ventura A, Pelicci PG (2002) Semaphorins: green light for redox signaling? Sci STKE 2002:PE44
Sierra JR, Corso S, Caione L, Cepero V, Conrotto P, Cignetti A, Piacibello W, Kumanogoh A, Kikutani H, Comoglio PM, Tamagnone L, Giordano S (2008) Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med 205:1673–1685
Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N (2009) PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 15:21–34
Albini A, Sporn MB (2007) The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer 7:139–147
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66:11238–11246
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4:71–78
Jubb AM, Strickland LA, Liu SD, Mak J, Schmidt M, Koeppen H (2012) Neuropilin-1 expression in cancer and development. J Pathol 226:50–60
Zhang S, Zhau HE, Osunkoya AO, Iqbal S, Yang X, Fan S, Chen Z, Wang R, Marshall FF, Chung LW, Wu D (2010) Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Mol Cancer 9:9
Mendonca DB, Mendonca G, Aragao FJ, Cooper LF (2011) NF-kappaB suppresses HIF-1alpha response by competing for P300 binding. Biochem Biophys Res Commun 404:997–1003
Shin DH, Li SH, Yang SW, Lee BL, Lee MK, Park JW (2009) Inhibitor of nuclear factor-kappaB alpha derepresses hypoxia-inducible factor-1 during moderate hypoxia by sequestering factor inhibiting hypoxia-inducible factor from hypoxia-inducible factor 1alpha. FEBS J 276:3470–3480
Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M (2008) NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453:807–811
van Uden P, Kenneth NS, Webster R, Muller HA, Mudie S, Rocha S (2011) Evolutionary conserved regulation of HIF-1beta by NF-kappaB. PLoS Genet 7:e1001285
Yang YH, Zhou H, Binmadi NO, Proia P, Basile JR (2011) Plexin-B1 activates NF-kappaB and IL-8 to promote a pro-angiogenic response in endothelial cells. PLoS ONE 6:e25826
Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM (2002) The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol 4:720–724
Ch’ng E, Tomita Y, Zhang B, He J, Hoshida Y, Qiu Y, Morii E, Nakamichi I, Hamada K, Ueda T, Aozasa K (2007) Prognostic significance of CD100 expression in soft tissue sarcoma. Cancer 110:164–172
Valente G, Nicotra G, Arrondini M, Castino R, Capparuccia L, Prat M, Kerim S, Tamagnone L, Isidoro C (2009) Co-expression of plexin-B1 and Met in human breast and ovary tumours enhances the risk of progression. Cell Oncol 31:423–436
Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, Risio M, Trusolino L, Weitz J, Schneider M, Mazzone M, Comoglio PM, Tamagnone L (2010) Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Invest 120:2684–2698
Fazzari P, Penachioni J, Gianola S, Rossi F, Eickholt BJ, Maina F, Alexopoulou L, Sottile A, Comoglio PM, Flavell RA, Tamagnone L (2007) Plexin-B1 plays a redundant role during mouse development and in tumour angiogenesis. BMC Dev Biol 7:55
Acknowledgments
The authors would like to thank Ernest Smith and Maurice Zauderer of Vaccinex, Inc., for providing the anti-SEMA4D antibody and offering technical support and Daniel Martin and Silvio Gutkind of the National Institute of Dental and Craniofacial Research, NIH, for contributing the head and neck cancer cell lines and assisting in the generation of shRNA lentiviruses. This work was supported by the National Cancer Institute grant R01-CA133162 to J.R.B.
Author information
Authors and Affiliations
Corresponding author
Additional information
The Editors-in-Chief have retracted this article following an investigation by the University of Maryland. The institution found problems in some figures. Due to these errors, the Editors-in-Chief have found that the results are no longer reliable.
About this article
Cite this article
Zhou, H., Binmadi, N.O., Yang, YH. et al. RETRACTED ARTICLE: Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression. Angiogenesis 15, 391–407 (2012). https://doi.org/10.1007/s10456-012-9268-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-012-9268-y
Keywords
Profiles
- Patrizia Proia View author profile