Abstract
Hospital-based outbreaks of severe acute respiratory syndrome (SARS) have once again highlighted the vulnerability of healthcare workers (HCWs). Use of personal respiratory protective equipment was the main method used by HCWs to avoid nosocomial transmission. This paper describes the technology used to evaluate the filtration efficiency of the half-face medical protection mask (N99), manufactured by Firmshield Biotechnology, against viral aerosol. Viral aerosol was generated and then sampled simultaneously with and without the test mask. This enables a percentage efficiency value to be calculated against test phage f2 aerosols (surrogates of viral pathogen aerosols). At the same time the mask filtration efficiency against NaCl particle aerosol was determined by use of TSI8130 equipment and face-fit factor was tested by use of TSI8020 equipment. The half-face medical protection mask (N99) evaluated by use of the viral aerosol had a filtration efficiency >99%. The mask filtration efficiency against NaCl particle aerosol was 99.634 ± 0.024% and it had a good face-fit factor. This half-face medical protection mask (N99) can protect the wearer from viral aerosol disease transmission. The test method can be used to assess filtration efficacy against viral aerosol of masks used for respiratory protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transmission of micro-organisms in the air plays a major role in some nosocomial infections, for example corona-type virus, Varicella Zoster, Mycobacterium tuberculosis, and Aspergillus niger (Griffiths et al. 2005). The epidemics of severe acute respiratory syndrome (SARS) in 2003, can be explained by aerosol transmission (Tang et al. 2006). With current concerns about a possible approaching influenza pandemic, the control of disease transmission via infectious air has become more important. Public health services and clinicians and practitioners will be confronted with a new need for infectious disease control. SARS posed a massive challenge because of the effect of nosocomial transmission on healthcare manpower and facilities, and the resources needed for controlling and preventing further spread (Tai 2006). Nishiyama showed that the risk of developing SARS was 12.6 times higher for individuals not using a mask than for those using a mask (Nishiyama et al. 2008). Consistent and proper use of a mask was shown to be crucial for constant protection against infection by SARS (Nishiyama et al. 2008; Low and Wilder-Smith 2005, Pei et al. 2006). Many types of masks were available and the different types offered very different levels of respiratory protection. It was difficult for non-specialist to assess the filtration efficiency of this equipment. Medical face mask materials tested by the standard method using Staphylococcus aureus aerosol as a bacterial model (ASTM 2007; BS EN 2006; SFDA 2004) were used to protect patients and surgical areas from contamination and not the wearers from the infectious aerosol. Half-face protection masks (N95 and N99) and full-face masks were mostly used in situations of high risk of aerosol transmission of diseases, but no standard testing method was used to evaluate filtration efficiency against viral aerosol. There is a requirement to develop and use standard methods to test such materials, using reliable aerosol-generating and sampling techniques, to assess their filtration efficacy against viral aerosols. This initial testing can be regarded as a primary “proof of principle” before respiratory protection materials are used. Aerosolization of a pathogenic virus requires a very high level of containment to prevent uncontrolled release. Because of aerosol safety issues involved with generation of high viral aerosol concentrations, the method of evaluation used a non-pathogenic virus. In this study, a viral model (bacteriophage f2) was used to test mask filtration efficiency against viral aerosol. A half-face medical protection mask (N99) manufactured by Firmshield Biotechnology was selected to evaluate filtration efficiency against viral aerosols and particle aerosols, and face-fit factor.
2 Materials and methods
2.1 Medical protection mask
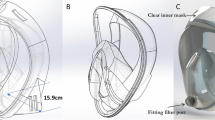
Half-face medical protection mask (N99) (18 cm × 9 cm, length × width) were provided by the manufacturer (Firmshield Biotechnology, China). The mask was made of N99 filtration material and used special face-fit technology. This N99 half-face medical protection mask is disposable personal protection equipment (PPE). The main function is to protect the wearer against infectious aerosols especially viral aerosols.
2.2 Physical particle test
Half-face medical protection mask (N99) filtration efficiency against NaCl particle aerosol was determined by use of the TSI8130 automated filter tester. Test flow rate was 85 L/min and the aerosol count median diameter (CMD) was 0.075 μm as required by the standard (SFDA 2003). TSI8130 can provide fast, reliable filter efficiency measurements up to 99.999%. Set the main regulator pressure (70 psi) and the holder regulator pressure (40 psi). Set the aerosol generator pressure (40 psi) and the make-up air flow rate (70 L/min), then warm-up for at least 30 min. Before testing mask filter efficiency we performed the salt generator media test using glass fiber filters (I.W. Tremont, NJ, USA) to check resistance and penetration against a standard graph then began testing the mask.
2.3 Face-fit factor
The face-fit factor of the tested half-face medical protection mask (N99) was determined by use of TSI8020 and N95 equipment. The TSI 8020 can successfully fit test class-100, class-99, and P3 disposable respirators, enabling fit testing using the respirator actually used by an individual. The mask can be fit tested by inserting a test probe through the filter material. The TSI model 8025-N95 probe kit includes disposable probes and insertion tools. In our test the fit factor pass level was set 150. In the USA, OSHA requires fit factor pass levels of 100 and 500 for half-face and full-face masks, respectively. Eight testing actions included normal breathing, deep breathing, head side to side, head up and down, talk out aloud, grimace, bend and touch toes, and normal breathing. The face-fit factor can range from 1–200; when the fit factor is more than 200 the result is 200+. Overall fit factors are automatically calculated by Fitplus software. The following equation is used to calculate the overall fit factor (FF): Overall \( {\text{FF}} = {\frac{n}{{{\frac{1}{{{\text{FF}}1}}}\,+\,{\frac{1}{{{\text{FF}}2}}}\,+\,{\frac{1}{{{\text{FF}}3}}}\,+\,\cdots\,+\,{\frac{1}{{{\text{FF}}n}}}}}}, \) where: FFx = fit factor for test cycle and N = number of test cycles (exercises). Each exercise includes an ambient sample, a mask sample, and then another ambient sample. The equipment measures respirator fit by comparing the concentration of microscopic particles outside the respirator with the concentration of particles that have leaked into the respirator. The fit factor is defined as the particle concentration outside the respirator divided by the particle concentration inside the respirator. A fit factor of 150 means that the air inside the respirator is 150 times as clean as the air outside.
2.4 Test organism
Bacteriophage f2 (ATCC 15766 B1) was used as model virus. Phage f2 (24 nm) is an icosahedron and one of the smallest viruses.
2.5 Preparation of test suspension
2.5.1 Preparation of viral suspension
An overnight culture at 37°C of E. coil (ATCC 15766) was inoculated into 10 ml nutrient broth in a conical flask and incubated for 4 h at 37°C with shaking. This logarithmic phase of culture was further inoculated (10 ml) into 100 ml nutrient broth and 1 ml f2 stock preparation (approximately 1.0 × 107 PFU/ml) was added. The culture was incubated overnight at 37°C. After incubation the culture was centrifuged (5,000g, 10 min) and the supernatant was filtered through a 0.22-μm filter.
2.5.2 Preparation of test suspension
The actual concentration of phage f2 in this preparation was determined by titration. A fresh preparation was made for each series of tests.
2.6 Titration of microorganisms
Phage f2 was titrated by preparing decimal dilutions of the sample in PBS. A culture of E. coli (0.5 ml) was added to 10 ml 0.7% agar while still molten, at a temperature of approximately 45°C. This was poured over a nutrient agar base and allowed to set. Each sample dilution (100 μl) was spotted on to the overlay and the plates were inoculated at 37°C for 16 h. The number of plaques was counted and the concentration expressed as plaque-forming units (PFU) per ml.
2.7 Size of aerosol particles
A multistage Andersen sampler (Kangjie Instruments, China) was connected to a cylinder aerosol chamber (113 cm2 × 50 cm, bottom area × height). Using a suspension containing phage f2. The test was performed at 28.3 L/min for 1 min at the control position (Fig. 1). The plates of bacteriophage f2 were coated by a layer of E. coli and semi-solid culture and incubated at 37°C for 16 h then the PFU on the plate were counted. The size of particle collected was determined from the presence of growth on each of the plates used (Holton and Webb 1997).
2.8 The test rig
The test rig is shown diagrammatically in Fig. 1. Filtered air can be drawn through the rig at 60 L/min by an air pump. The DV40 nebulizer was supplied with a 30-mL suspension of the phage f2. The principle of the DV40 nebulizer was similar to that of the collision nebulizer. The compressed air sheared the liquid into droplets. The larger fraction of the droplets was removed and the smaller fraction was ejected by the nebulizer. Phage f2 was aerosolized by applying compressed air to the nebulizer at 10 L/min. The aerosolized phage f2 aerosol was passed into the aerosol chamber and mixed uniformly in the chamber. At the downstream end of the chamber, another length of duct was connected, linked to the fan units via a back-up high-efficiency particulate air filter assembly that was designed to prevent the escape of any viral aerosol into the environment. Multistage Andersen samplers were used to sample the air at two positions. The first was the control position, to obtain a control sample, and the second was the test position, to obtain a test sample, to determine the viral aerosol concentration before and after mask filtration, respectively. When testing the half-face medical protection mask the flow was 28.3 L/min and sampling time was 1 min in the control position; in the test position the flow was 28.3 L/min and the sampling time was 2 min. The collecting agars were cultured and the plaque numbers counted. The filtration efficiency was determined from the aerosol concentration before and after the tested sample.
2.9 Culture the collected samples
Collected samples of phage f2 were coated by a layer of 0.5 ml E. coli and 10 ml semi-solid culture and incubated at 37°C for 16 h. Plaque numbers on the plate were then counted. The number of plaques on each plate was revised as reference (Andersen 1958).
2.10 Calculation of performance efficiency
By taking pre-mask and post-mask viral aerosol samples with the sampling device, the method enables simultaneous measurement of viral aerosol concentration before and after filtration. The percentage efficiency of the test mask was calculated by use of the following formula, where A is the concentration of viral aerosol challenging the mask and B is the concentration of viral aerosol after filtration. Phage f2 aerosol was determined as PFU/m3 \( Efficiency \, (\% ) = \left( {1 - \frac{B}{A}} \right) \times 100\% \).
3 Results
3.1 Physical particle test
The results for filtration efficiency of the tested masks against physical aerosol particles are shown in Table 1. Average filtration efficiency of the mask was 99.634 ± 0.024%.
3.2 Face-fit factor
The face-fit factors of the masks are shown in Table 2. The overall fit factor pass level was set at 150. The overall fit factor for all ten test persons was above 150. The tested masks had good face-fit factors for different persons. The measurement provided by the equipment is an assessment of mask fit during a fit test only. Mask fit at other times will vary. The fit factor value is not intended for use in calculating an individual’s actual exposure to hazardous substances.
3.3 Aerosol particle sizes
The nebulizer produced viral aerosol particles between 0.6 and 7.0 μm in the following percentages: 0.6–1.0 μm 45.2%, 1.1–2.0 μm 45.2%, 2.1–3.3 μm 9.5%, 3.4–4.7 μm 0.07%, 4.8–7.0 μm 0.05%, >7 μm 0.02%. CMD of phage f2 aerosol was 1.20 μm.
3.4 Filtration efficiency of half-face medical protection mask
Five new masks samples were selected to test filtration efficiency against viral aerosols. The results from filtration efficiency tests against viral aerosol are shown in Table 3. A Multistage Andersen sampler was used to collect air after filtration by the N99 mask; the flow rate was 28.3 L/min. The sampling time was set at 2 min because prolonged tests may have caused excessive drying of agar and loss of viral viability, so the testing limit was 17 PFU/m3. Because there were no phage f2 plaques on the collected agar of the tested samples, the result was <17 PFU/m3. For all the masks tested filtration efficiency for phage f2 aerosol was >99%.
4 Discussion
Workers, primarily those in the health care professions involved in treating and caring for individuals injured or sick, and patients can be exposed to biological aerosols capable of transmitting disease. These diseases, which may be caused by a variety of microorganisms, can pose significant risks to life and health. This was tragically highlighted during the international outbreak of severe acute respiratory syndrome (SARS) in 2003 with attack rates of more than 50% in HCW (Wilder-Smith and Low 2005). Failure to implement appropriate barrier precautions was responsible for most nosocomial transmissions. SARS focused attention on the adequacy of, and compliance with, infection-control practices in preventing airborne and droplet-spread transmission of infectious agents (Gamage et al. 2005). Even if SARS does not re-emerge, the experience gained from SARS was valuable in preparing for threats of bioterrorism or a global influenza virus pandemic. The right choice of personal protective equipment (PPE) is critical. The selection of respiratory protection levels to be used against a biological agent should be based on infectious risk assessment. Surgical masks protect the patient and surgical area from contamination. They prevent particles (droplets) being expelled. They protect patients in surgery from these particles being transferred to the operative site. They were not suitable when the risk of infection by aerosol transmission of diseases was high. Medical protection masks (N95 and N99) were mostly used when there was high risk of aerosol transmission of diseases.
Because of the special aerosol properties of bioaerosols, much equipment used for protection against aerosol transmission of diseases was required to undergo bioaerosol protection testing (Rengasamy et al. 2004). But there was no standard method for testing the filtration efficiency against viral aerosol of equipment used for high-risk respiratory protection. Several studies have reviewed the role of respiratory protective devices in the control of TB in health-care settings (Hodous and Coffey 1994; Jarvis et al. 1995, Schaefer 1997; McCullough and Brosseau 1999). Studies on respiratory protection against TB were carried out with nonpathogenic bacteria having physical characteristics similar to those of M. tuberculosis (Chen et al. 1994; Qian et al. 1998; Brosseau et al. 1997). In our study, half-face medical protection masks (N99) were selected to test filtration efficiency against viral aerosol by using a viral model phage f2. Efficiencies of the masks against viral aerosol were determined. Scientifically rigorous testing of the filtration efficiency of respiratory protection intended for use is the first step in investigating the potential benefits of using them to reduce the incidence of nosocomial infection. This study dealt with the primary concern of mask filtration efficiency against viral aerosol and some important characteristics such as face-fit factor and filtration efficiency against particle aerosols, but excluded other physical characteristics such as skin hypersusceptibility, maintenance, and storage. In our study the half-face medical protection mask (N99) had high filtration efficiency—>99% against viral aerosol, >99% against particle aerosol, and a good face-fit factor, which can protect wearers against the aerosol transmission. The results of the viral aerosol test were accordance with results for physical particles. Phage f2 was used as surrogate viral aerosol, similar to other researchers (Chen et al. 2008). The surrogate phage f2 was used in place of the pathogenic virus because of aerosol safety issues. Particles in the 1.1–2.1 μm size range lead to the greatest risk of airborne infection (Ko and Burge 2007). In our study CMD of phage f2 aerosol was 1.20 μm.
Research on respiratory protection against biological agents is important for addressing major concerns such as occupational safety and terrorist attack. But test methods and procedures are not fully developed and described in the literature. This limits the amount of cross-comparison of results that can be validly performed. Furthermore, the conditions used for the described test methods and procedures are not standardized. For example, a number of flow rates were used in the various studies, and this can substantially affect penetration through the filters (Rengasamy et al. 2004). Finally, the paucity of literature on various aspects of respiratory protection against bioaerosols is a limiting factor in drawing conclusions. This test method has been designed to introduce an aerosol challenge to the test specimens at a flow rate of 28.3 L/mm. This flow rate is within the range of normal respiration and within the limitations of the samplers. This test method was used to measure the viral aerosol filtration efficiency of respiratory protection equipment, using the ratio of aerosol concentration before and after the test samples to determine filtration efficiency of respiratory protection materials. It is a quantitative method that enables filtration efficiency for respiratory protection materials to be determined. The flow rate can be adjusted if other flow rate air samplers are used and the aerosol concentration can be adjusted by dilution of the air, so the method can be used to evaluate the filtration efficiency of respiratory protection materials against viral aerosol with larger test flow rates.
References
Andersen, A. A. (1958). New sampler for the collection, sizing, and enumeration of viable particles. Journal of Bacteriology, 76, 471–484.
ASTM international. (2007). F2101–07 standard test method for evaluating the bacterial filtration efficiency (BFE) of medical face mask materials, using a biological aerosol of Staphylococcus aureus. West Conshohocken, PA: ASTM international.
Brosseau, L. M., McCullough, N. V., & Vesley, D. (1997). Mycobacterial aerosol collection efficiency of respirator and surgical mask filters under varying conditions of flow and humidity. Applied occupational and environmental, 12, 435–445.
BS EN. (2006). BS EN 14683:2005 surgical masks—requirements and test methods. London, UK: British Standard Institution.
Chen, S. K., Vesley, D., Brosseau, L. M., & Vincent, J. H. (1994). Evaluation of single-use masks and respirators for protection of health care workers against mycobacterial aerosols. American Journal of Infection Control, 22, 65–74.
Chen, Z. B., Huang, H. Y., & Zhang, C. W. (2008). Comparative study of the resistance of bacteriophageT4, PhiX174, MS2 and f2 to gamma radiation. Zhonghua Yi Xue Za Zhi, 88, 198–201.
Gamage, B., Moore, D., Copes, R., Yassi, A., & Bryce, E. (2005). Protecting health care workers from SARS and other respiratory pathogens: a review of the infection control literature. American Journal of Infection Control, 33, 114–121. doi:10.1016/j.ajic.2004.12.002.
Griffiths, W. D., Bennett, A., Speight, S., & Parks, S. (2005). Determining the performance of a commercial air purification system for reducing airborne contamination using model micro-organisms: A new test methodology. Journal of Hospital Infection, 61, 242–247. doi:10.1016/j.jhin.2005.03.004.
Hodous, T. K., & Coffey, C. C. (1994). The role of respiratory protective devices in the control of tuberculosis. Occupational Medicine, 9, 631–657.
Holton, J., & Webb, A. R. (1997). An evaluation of the microbial retention performance of three ventilator-circuit filters. Intensive Care Medicine, 20, 233–237. doi:10.1007/BF01704708.
Jarvis, W. R., Bolyard, E. A., Bozzi, C. J., Burwen, D. R., Dooley, S. W., Martin, L. S., et al. (1995). Respirators, recommendations, and regulations: The controversy surrounding protection of health care workers from tuberculosis. Annals of Internal Medicine, 122, 142–146.
Ko, G., & Burge, H. (2007). Removal of Serratia marcescens aerosols using an electrostatic precipitator. Journal of microbiology and biotechnology, 17, 1622–1628.
Low, J. G., & Wilder-Smith, A. (2005). Infectious respiratory illnesses and their impact on healthcare workers: A review. Annals of the Academy of Medicine, Singapore, 34, 105–110.
McCullough, N. V., & Brosseau, L. M. (1999). Selecting respirators for control of worker exposure to infectious aerosols. Infection Control and Hospital Epidemiology, 20, 136–144.
Nishiyama, A., Wakasugi, N., Kirikae, T., Quy, T., Ha le, D., Ban, V. V., et al. (2008). Risk factors for SARS infection within hospitals in Hanoi, Vietnam. Japanese Journal of Infectious Diseases, 61, 388–390.
Pei, L. Y., Gao, Z. C., Yang, Z., Wei, D. G., Wang, S. X., Ji, J. M., et al. (2006). Investigation of the influencing factors on severe acute respiratory syndrome among health care workers. Beijing Da Xue Xue Bao, 38, 271–275.
Qian, Y., Willeke, K., Grinshpun, S. A., Donnelly, J., & Coffey, C. C. (1998). Performance of N95 respirators: Filtration efficiency for airborne microbial and inert particles. American Industrial Hygiene Association Journal, 59, 128–132. doi:10.1080/15428119891010389.
Rengasamy, A., Zhuang, Z., & BerryAnn, R. (2004). Respiratory protection against bioaerosols: Literature review and research needs. American Journal of Infection Control, 32, 345–354. doi:10.1016/j.ajic.2004.04.199.
Schaefer, J. A. (1997). Respiratory protection in the health care setting. Occupational Medicine, 12, 641–654.
State food, drug administration(SFDA). (2003). GB19083–2003 Technical requirements for productive face mask for medical use. Beijing, China: Standards press of China.
State food, drug administration(SFDA). (2004). YY0469–2004 Technical requirements for surgical mask. Beijing, China: Standards press of China.
Tai, D. Y. (2006). SARS: How to manage future outbreaks? Annals of the Academy of Medicine, Singapore, 35, 368–373.
Tang, J. W., Li, Y., Eames, I., Chan, P. K. S., & Ridgway, G. L. (2006). Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. Journal of Hospital Infection, 64, 100–114. doi:10.1016/j.jhin.2006.05.022.
Wilder-Smith, A., & Low, J. G. (2005). Risk of respiratory infections in health care workers: Lessons on infection control emerge from the SARS outbreak. Southeast Asian Journal of Tropical Medicine and Public Health, 36, 481–488.
Acknowledgments
The authors thank Dr Sun Zhenhai for helpful suggestions for this work. This work was supported by National science and technology support program of China (No. 2008BAI62B05) and National importance infectious disease program of China (No. 2009ZX10004-501).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wen, Z., Lu, J., Li, J. et al. Determining the filtration efficiency of half-face medical protection mask (N99) against viral aerosol. Aerobiologia 26, 245–251 (2010). https://doi.org/10.1007/s10453-010-9160-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-010-9160-4