Abstract
Oxide material of a new type, ZrO2·SiO2 (raw and carbonyl-grafted), was used as support in the immobilization of aminoacylase from Aspergillus melleus. The ZrO2·SiO2 was synthesized via sol–gel method. The obtained material was additionally modified with glutaraldehyde. Various physicochemical analyses were used to confirm the effectiveness of the modification and immobilization processes, including Fourier transform infrared spectroscopy, laser Doppler velocimetry and low-temperature N2 sorption. The immobilization process was performed within 3 h using different concentrations of enzyme solution (1, 3, 5 and 7 mg/mL), and the Bradford method was used to determine the quantity of immobilized enzyme. The resulting biocatalytic systems were then used as catalysts in the hydrolysis of different N-acetyl-dl-amino acids (leading to l-methionine, l-cysteine, l-serine and l-tryptophan). Based on this reaction the apparent and relative catalytic activities were determined. The highest activity of the immobilized enzyme was attained in the synthesis of l-methionine (the apparent activities of aminoacylase immobilized on raw and carbonyl-grafted ZrO2·SiO2 were 4112 and 4947 U/g, respectively). Furthermore, the effect of pH and temperature on catalytic activity, as well as the storage stability and reusability of the prepared biocatalytic systems were determined. Aminoacylase immobilized on carbonyl-grafted ZrO2·SiO2 retains 85% of its initial activity after 30 days of storage and 70% after five reaction cycles.
Similar content being viewed by others
1 Introduction
The fabrication of novel types of biocatalytic systems has gained great importance in recent years. Such systems are most commonly obtained via the immobilization of enzymes on various supports. There are several important aspects that should be considered when selecting an appropriate support for enzyme immobilization. First of all the support should be biocompatible and should have active functional groups on its surface (Wu et al. 2013). These groups should be stable in the reaction conditions, should exhibit low steric hindrance, and should enable combination with the amino acids groups present in the enzyme molecule. Additionally, the support should have good thermal, chemical and mechanical stability, and the surface should be hydrophilic. The support should also be insoluble in the reaction conditions. The most important factors are the ability to immobilize enzymes on the chosen material, and its price (Cowana and Fernandez-Lafuente 2011; Homaei et al. 2013). Inorganic hybrid materials exhibit most of the properties mentioned above, and offer higher thermal and mechanical stability than organic materials (Jesionowski et al. 2014). Moreover, these materials exhibit microbial resistance, because they do not serve as a matrix for the growth of bacteria and fungi, unlike more popular organic materials such as cellulose, polyamine and polydextrans. The inorganic materials do not change their structure in different pH and temperature conditions or in organic solvents, in contrast to organic materials and polymers. They have a higher modulus of elasticity than organic polymers. These materials have a highly hydrophilic surface, because of the hydroxyl groups present on it. That is why it is possible to modify their surface, promoting the creation of relatively stable interactions between enzymes and the support. Furthermore, many inorganic materials are characterized by high rigidity and porosity (Mateo et al. 2007).
The many different supports commonly used in the immobilization of enzymes include inorganic oxides such as SiO2 (Kolodziejczak-Radzimska 2017; Jin et al. 2018), TiO2 (Zivkovic et al. 2016; Haghighi et al. 2017) and ZrO2 (Masuda et al. 2014), minerals such as bentonite (Cengiz et al. 2012) and halloysite (Zhai et al. 2010; Chao et al. 2013), and carbon materials (Mohiuddin et al. 2014; Wu et al. 2017; Das et al. 2018). These materials have been used in the immobilization of lipase (Cai et al. 2016; Zdarta et al. 2015; Kolodziejczak-Radzimska et al. 2018b; Heater et al. 2018), cysteine (Xiao et al. 2010; Noori et al. 2016), urease (Piccinini et al. 2017; Tak et al. 2017), α-amylase (Demkina et al. 2017), tyrosinase (Abdollahi et al. 2017), laccase (Pogorilyi et al. 2017) and other enzymes. As it can be seen a number of enzymes have been immobilized onto different materials. Additionally, this biocatalytic systems found their application in the treatment of industrial wastewaters. For instance, they were successfully applied for dye removal, degradation of pharmaceuticals, xenobiotics, and treatment of industrial effluents (Pylypchuk et al. 2018; Antecka et al. 2018).
Besides typical inorganic materials, interesting properties are offered by inorganic hybrids and composites and by oxide systems (Zdarta et al. 2018). These type of materials are synthesized from both organic and inorganic precursors. They are formulated to ensure the high stability of the resulting systems and good affinity to enzymes. Additionally, they contain numerous characteristic groups which show affinity to the chemical groups present in the protein structure (Ambrogio et al. 2011). The presence of functional groups enables the formation of covalent bonds with biomolecules, and ensures the good reusability and operational stability of the obtained biocatalytic systems.
Among the materials offering interesting properties are zirconia/silica oxide systems. These hybrid combine the properties of silica and zirconium oxide (Del Angel-Lopez et al. 2015), the most important of which are good stability, high strength, high fracture toughness, excellent wear resistance, high hardness and excellent chemical resistance (Jesionowski and Krysztafkiewicz 2001; Liang et al. 2009; Shibli et al. 2010; Klapiszewski et al. 2014). Due to its numerous advantages, the sol–gel process is commonly used for the production of nanostructured materials. This method makes it possible to obtain products that are homogeneous in terms of chemical composition, characterized by high purity and controlled physicochemical parameters. These types of materials are also useful in environmental applications, including the removal of various type of inorganic and organic impurities from water systems, via either adsorption or photocatalysis (Vaizogullar et al. 2015; Santos et al. 2014; Ciesielczyk et al. 2018).
In this study, for the first time, the ZrO2·SiO2 material was used as a support in the immobilization of aminoacylase from Aspergillus melleus. The simple, conventional adsorptive method has been used for enzyme immobilization. The adsorptive method as compared to other immobilization techniques is more simple and enables higher mobility of the enzyme, which in many cases results in a significant enzyme activity. The ZrO2·SiO2 was modified by glutaraldehyde to introduce carbonyl groups onto the surface. The products were analyzed by a number of techniques (including Fourier transform infrared spectroscopy, laser Doppler velocimetry and low-temperature N2 sorption). The obtained biocatalytic systems were used as catalysts in the synthesis of selected amino acids (l-methionine, l-cysteine, l-serine and l-tryptophan).
2 Materials and methods
2.1 Materials
Solutions of tetraethyl orthosilicate (TEOS) and zirconium isopropoxide (TPZ), 25% ammonia solution (NH3aq.) and ethyl alcohol (EtOH) were used in the synthesis of ZrO2·SiO2 as precursors, promotor of hydrolysis and solvent, respectively. Glutaraldehyde (GA) was used as a modifier. Aminoacylase from Aspergillus melleus (AAM), N-acetyl-l-methionine (AcMet), l-methionine (Met), N-acetyl-dl-serine, l-serine, N-acetyl-l-cysteine, l-cysteine, N-acetyl-dl-tryptophan, l-tryptophan, Bradford reagent, ninhydrin reagent, monobasic sodium phosphate (NaH2PO4) and dibasic sodium phosphate (Na2HPO4) were used in the immobilization process. All of the materials were purchased from Sigma-Aldrich® (St Louis, MO).
2.2 Synthesis, modification and characterization of ZrO2·SiO2
The ZrO2·SiO2 oxide system was synthesized via a sol–gel method according to methodology presented in previously published article (Ciesielczyk et al. 2018). The product was obtained from organic precursors of silica (TEOS) and zirconia (TPZ). The molar ratio of the reagents was established as to obtain zirconia:silica ratio of 1:1. The 25% ammonia solution was used as promoter of hydrolysis and ethanol as a solvent. Resulting alcogel was gently dried at 105 °C within 12 h in order to slowly remove the solvent and enable the formation of well-developed porosity, so important in immobilization process. The material was thoroughly characterized with respect to parameters of the porous structure, type of surface functional groups (FTIR analysis) as well as electrokinetic stability (zeta potential measurements). Synthesized sample was furthermore modified with glutaraldehyde (5% solution). In this case the 1 g of ZrO2·SiO2 oxide material was stirred with the glutaraldehyde solution, and then filtered and dried (60 °C, 2 h). The resulting sample was labelled as ZrO2·SiO2_GA.
A series of physicochemical evaluations was performed. Firstly, FTIR spectra were obtained using a Vertex 70 spectrometer (Bruker). Samples were analyzed in the form of tablets, made by pressing a mixture of anhydrous KBr (ca. 0.25 g) and 1 mg of the tested substance in a special steel ring, under a pressure of 10 MPa. The zeta potential of the obtained materials was determined as a function of pH, using a Zetasizer Nano ZS equipped with an autotitrator (Malvern Instruments Ltd., UK), which enables estimation of electrophoretic mobility, and indirectly of the zeta potential, based on laser Doppler velocimetry measurments. The value of the zeta potential was calculated from the Smoluchowski equation. Low-temperature N2 sorption was also applied. The surface area (ABET) and mean pore diameter (Sp) were determined using an ASAP 2020 instrument (Micromeritics Instrument Co., USA). The surface area was determined by the multipoint BET (Brunauer–Emmett–Teller) method, using data on adsorption as a function of relative pressure (p/p0).
2.3 Immobilization of aminoacylase from Aspergillus melleus
Aminoacylase was immobilized on the ZrO2·SiO2 materials (raw and carbonyl-grafted ZrO2·SiO2) via an adsorption method. The enzyme solution (1, 3 and 7 mg/mL, 0.2 M solution of phosphate buffer (PBS) at pH = 7) was stirred with the support (0.5 g) for 3 h at ambient temperature. The obtained biocatalytic systems were filtered and washed. The quantity (P) of aminoacylase immobilized on the ZrO2·SiO2 (raw and carbonyl-grafted) and the immobilization yield (IY) were analyzed using the Bradford method and calculated from Eqs. (1–2):
where C0 and C1 denote the concentration of the enzyme (mg/mL) in solution before and after immobilization respectively, V is the volume of solution (mL), and m is the mass of support (g). Desorption of the immobilized aminoacylase was evaluated over a time of 3 h using phosphate buffer at pH = 7. For this purpose, both ZrO2·SiO2 and carbonyl-grafted ZrO2·SiO2 were dispersed in PBS solution. After the specified period of time, the efficiency of desorption (D) was evaluated based on the Bradford method and calculated from Eq. (3):
where C2 and C1 denote the concentration of the enzyme (mg/mL) in solution before and after desorption respectively.
2.4 Enzyme assay
The catalytic activity is a very important parameter determining the possible use of an enzyme as a catalyst. Aminoacylase catalyzes the asymmetric hydrolysis of N-acetyl-dl-amino acids to l-amino acids and unhydrolyzed N-acetyl-d-amino acids (Fig. 1).
The free and immobilized aminoacylase were used to catalyze the hydrolysis of N-acetyl-l-methionine, N-acetyl-l-cysteine, N-acetyl-dl-serine and N-acetyl-dl-tryptophan to l-methionine, l-cysteine, l-serine and l-tryptophan, respectively. The apparent activity (AAp, U/g) of AAM was measured using the colorimetric ninhydrin method, and was defined as the quantity of enzyme which hydrolyzed 1 µmol of l-amino acid per minute, per 1 g of biocatalyst. The release of the product was observed at 570 nm (using a JASCO V650 spectrophotometer, Japan). Additionally, the relative activity (AR, %) was calculated from Eq. (4):
2.5 Evaluation of the stability of free aminoacylase and obtained biocatalytic systems
The effect of temperature (30–70 °C) and pH (4–9) on the catalytic activity of the obtained biocatalytic systems (aminoacylase immobilized on raw and carbonyl-grafted ZrO2·SiO2) was examined based on the reaction illustrated in Fig. 1. The storage stability (after 30 days) and reusability (5 cycles) were also evaluated.
3 Results and discussion
3.1 Physicochemical characterization of supports and biocatalytic systems
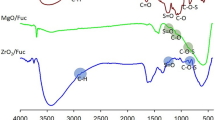
The first stage of physicochemical evaluation involved infrared spectral analysis. Figure 2 shows the FTIR spectra of the supports, free aminoacylase, and aminoacylase immobilized on ZrO2·SiO2 and ZrO2·SiO2_GA. The most important features of the ZrO2·SiO2 surface (Fig. 2a) include O–H stretching vibrations (between 3200 and 3700 cm−1), deformational vibrations of –OH groups (1629 cm−1) arising from physically adsorbed water, and asymmetric vibrations of Si–O–Si and Zr–O–Si between 1200 and 950 cm−1; the bands at 675–600 cm−1 indicate the presence of Si–O and Zr–O bonds (Ciesielczyk et al. 2018; Rahulan et al. 2013). Additionally, the FTIR spectrum of carbonyl-grafted ZrO2·SiO2 contained bands corresponding to –CH and C=O stretching vibrations at 2850 and 1530 cm−1, respectively.
The FTIR spectrum of free aminoacylase from Aspergillus melleus (Fig. 2b) contains a wide range of signals. The band at 3430 cm−1 indicates the presence of hydroxyl groups in the enzyme structure. Close to 2935 cm−1 is a band corresponding to stretching vibrations of –C–H bonds. Important features include the bands at 1652 and 1540 cm−1 corresponding to vibrations of amide I and II bonds, and a peak at 1120 cm−1 generated by stretching vibrations of C–O bonds present in the protein structure.
Figure 2c and d show the FTIR spectra of aminoacylase immobilized on raw and carbonyl-grafted ZrO2·SiO2 (ZrO2·SiO2_GA). The presence of amide bonds (amide I on the spectrum of aminoacylase immobilized on ZrO2·SiO2, and amide I and II on the spectrum of aminoacylase immobilized on ZrO2·SiO2_GA) between 1450 and 1630 cm−1 indirectly confirm the effectiveness of the immobilization process. The intensity of amide bond signals increased with increasing concentration of enzyme solution; this is probably associated with the quantity of immobilized enzyme.
The surface charges of the free enzyme and of the support with and without enzyme were determined based on values of the zeta potential as a function of pH (Fig. 3). The ZrO2·SiO2 zeta potential values give a high potential in a range from 38 to − 48 mV, in the whole analyzed pH range (2–10). The isoelectric point (IEP) for this sample is 4.1. For the free aminoacylase from Aspergillus melleus the zeta potential value ranges from 10 to − 35 mV, with an isoelectric point of 3.4. The ZrO2·SiO2 containing immobilized enzyme (ZrO2·SiO2_AAM) has a more negative potential than the raw materials. In this case the zeta potential is negative (between − 10 and − 60 mV) in the whole analyzed pH range.
Modification of ZrO2·SiO2 with glutaraldehyde led to a change in the value of the isoelectric point. The IEP for this sample was recorded at pH = 5.7. On the other hand, the zeta potential values for carbonyl-grafted ZrO2·SiO2 lie in a similar range (from 35 to − 50 mV in the analyzed pH range) as in case of raw ZrO2·SiO2. Immobilization of the enzyme onto carbonyl-grafted ZrO2·SiO2 (ZrO2·SiO2_GA_AAM) led also to some changes in the zeta potential values calculated for varying pH, as compared with the raw support. The zeta potential for sample ZrO2·SiO2_AG_AAM ranged from 20 to − 50 mV. Moreover, a shift in the IEP value (pH = 5.1) for this sample was observed.
The changes in zeta potential after immobilization probably results from differences in the surface charge of the materials, as well as interactions between the enzyme and support (Coutinho et al. 2018). These phenomena may be related to the electrostatic interactions (NH3+, COO−, O−). The covalent bonds generated between the groups present in the enzyme (–NH2) and in the modified support (–C=O) are also of great importance. This fact was confirmed by the FTIR analysis. Besides these, the binding may also be supported by hydrophobic interactions and van der Waals interactions (Mehde et al. 2018). Additionally, the immobilization of the enzyme onto ZrO2·SiO2 oxide system shifts the slipping plane a little bit stronger and the zeta potential is more negative (Wisniewska et al. 2015; Szewczuk-Karpisz and Wisniewska 2018).
Based on the FTIR and zeta potential analysis, a probable mechanism of immobilization of AAM onto carbonyl-grafted ZrO2·SiO2 was proposed (Fig. 4).
The next stage of physicochemical evaluation concerned the influence of the immobilization process on porous structure parameters, such as surface area and pore size (Table 1). Figure 5 presents the nitrogen adsorption/desorption isotherms of the supports ZrO2·SiO2 and ZrO2·SiO2_GA. The obtained isotherms were classified as type II, and their shape suggests that there exist only mesopores between small particles (Ciesielczyk et al. 2018). The unmodified ZrO2·SiO2 has the highest surface area (ABET = 276 m2/g). The average pore size of this sample is 5.3 nm. Modification of zirconia/silica oxide system with glutaraldehyde caused a significant decrease in surface area (to 194 m2/g), but the average pore size increased slightly (to 6.5 nm) due to incorporation of modifier molecules. The surface area of oxide material decreased after modification with GA, which is probably associated with blocking of the active sites (in this case hydroxyl groups present on the ZrO2·SiO2 surface) by the carboxyl group from glutaraldehyde.
The immobilization process leads to a decrease in the values of the porous structure parameters, which may serve as an indirect indication of the effectiveness of immobilization. This is evidenced by the smaller surface area values (60–68 m2/g) and pore sizes (2.1 nm) as compared with the raw supports. The reduction in the pore size after immobilization is probably associated with the immobilization of enzyme inside the pores and with the blocking of pore spaces (Forde et al. 2010).
The data given in Table 2 show that the quantity of immobilized aminoacylase increases with an increase in the enzyme concentration, reaching maximum values of 745 mg/g (for 7 mg/mL aminoacylase immobilized onto ZrO2·SiO2) and 746 mg/g (for 7 mg/mL aminoacylase immobilized onto ZrO2·SiO2_GA). The same dependence was observed for immobilization yield, which also increased with increasing concentration of the enzyme, reaching highest values of 99.4–99.6% for both supports. The obtained systems exhibited high stability—desorption of the enzyme did not exceed 0.2%. This is closely related to the efficiency of adsorption of the enzyme and its various interactions with the zirconia/silica oxide system.
The obtained biocatalytic systems (ZrO2·SiO2_AAM and ZrO2·SiO2_GA_AAM) were used as biocatalysts in the hydrolysis of certain N-acetyl-l-amino acids (Tables 2 and 3). Based on this reaction, the catalytic activity of the immobilized aminoacylase was determined. N-acetyl-l-methionine is most often used as a substrate in hydrolysis reactions. Table 3 contains the values of apparent (AAp) and relative (AR) activity obtained for aminoacylase (at concentrations of 1, 3 and 7 mg/mL) immobilized onto unmodified and modified ZrO2·SiO2. These data show that apparent and relative activity increased with increasing concentration of aminoacylase. The immobilized aminoacylase retains high relative activity (AR = 45% for ZrO2·SiO2_AAM_7 mg and AR = 54% for ZrO2·SiO2_GA_AAM_7 mg) as compared with the activity of the native enzyme. Aminoacylase immobilized onto carbonyl-grafted ZrO2·SiO2 was found to exhibit better catalytic activity. This may be related to the formation of stable bonds between carbonyl groups from the support and amine groups from the enzyme.
Catalytic tests were also performed using other amino acids derivatives (N-acetyl-l-cysteine, -dl-serine and -dl-tryptophan). The results, given in Table 4, show that the aminoacylase immobilized onto ZrO2·SiO2 and ZrO2·SiO2_GA can also be used to catalyze the synthesis reactions of l-cysteine, dl-serine and dl-tryptophan, although the obtained values of apparent and relative activity are smaller than those presented in Table 3. The values of relative activity for the immobilized enzyme are higher in the synthesis of l-cysteine and l-tryptophan (34% and 28% respectively for ZrO2·SiO2_AAM, 22% and 38% for ZrO2·SiO2_GA_AAM). This fact may be related to the structure of the amino acids used. In the literature, besides the standard derivative of methionine, N-acetyl-l-theanine has also been used to determine the enzymatic activity of immobilized aminoacylase (Li et al. 2015). In that study the immobilized aminoacylase (on electrospun nanofibrous membrane) had a relative activity of 50%.
The last stage of the research involved determination of the influence of pH and temperature on the activity of free and immobilized aminoacylase, in addition to storage stability and reusability. The results are presented in Figs. 6 and 7. Free aminoacylase retains high activity in the pH range 6–8 (activity above 70%) and in the temperature range 40–50 °C (activity above 70%). Below pH = 6 and 40 °C the relative activity rapidly decreases; the same is observed above pH = 8 and 50 °C. The immobilized enzyme, however, exhibits good activity in the whole analyzed pH range and temperature range. Better catalytic activity was observed for aminoacylase immobilized onto carbonyl-grafted ZrO2·SiO2, which is probably related to the nature of the bonds formed (covalent bonds) between the enzyme and the support. In this case the immobilized enzyme exhibited activity above 50% in the whole analyzed pH range and activity above 60% in the temperature range 30–60 °C. Aminoacylase immobilized on raw ZrO2·SiO2 (ZrO2·SiO2_AAM) also retains good activity (above 40%) at pH = 5–9, but the activity of this biocatalytic system is above 60% only in the temperature range from 30 to 50 °C. Above 50 °C the immobilized AAM exhibits practically no activity. Similar findings were reported by Xiao et al. (2006) (acylase immobilized on hollow silica nanotubes) and Pang et al. (2016) (laccase immobilized on a micro-mesoporous Zr-metal organic framework).
The storage stability and reusability of the obtained systems were also investigated, and the results are shown in Fig. 7. The biocatalytic systems retained enzymatic activity after 30 days of storage and after five reaction cycles. The system ZrO2·SiO2_GA_AAM has better catalytic properties than ZrO2·SiO2_AAM. Aminoacylase immobilized onto ZrO2·SiO2_GA retains approximately 85% of its initial activity after 30 days, and 70% after five cycles. This is probably related to the more stable covalent bonds formed between the enzyme and the support.
In summary, the results of the study have shown that immobilization process of aminoacylase onto zirconia/silica oxide systems (ZrO2·SiO2 and ZrO2·SiO2_GA) was successfully carried out. The strength of the interactions between enzyme and support may be due to covalent bonds. These bonds are probably formed between carbonyl and amino groups, present on the support surface and in the enzyme structure respectively. One of the most important advantages of an immobilized enzyme is its reusability (Zivkovic et al. 2015). Table 5 gives the relative activity after five cycles of various biocatalytic systems. In previous studies the enzymes were immobilized on materials consisting of silica or zirconia only, unlike the combined zirconia/silica systems used in the present study. The data in Table 5 show that enzymes immobilized on silica or zirconia retain only 40–50% of their initial activity. Only one study found that the relative activity of an enzyme immobilized on a silica or zirconia support reached 90%. This is probably related to the immobilization method used or the structure of the enzyme in question (lipase).
4 Conclusion
Aminoacylase from Aspergillus melleus has been successfully immobilized onto raw and carbonyl-grafted ZrO2·SiO2. Standard Bradford analysis was performed, in addition to a number of physicochemical techniques, including Fourier transform infrared spectroscopy, laser Doppler velocimetry and low-temperature N2 sorption, to investigate the effectiveness of the immobilization process. Changes observed in the FTIR spectra and variations in zeta potential are probably caused by differences in the surface conditions of the particles, as well as interactions involving other particles and ions in the solution. Based on all of the analyses, a mechanism has been proposed for the reaction of immobilization of aminoacylase onto carbonyl-grafted ZrO2·SiO2, primarily involving electrostatic and covalent interactions. Moreover, it has been shown that aminoacylase immobilized on raw and carbonyl-grafted ZrO2·SiO2 can be used as a biocatalyst in the synthesis of amino acids (l-methionine, l-cysteine, l-serine and l-tryptophan). Aminoacylase from Aspergillus melleus immobilized on carbonyl-grafted ZrO2·SiO2 was less sensitive to temperature and pH changes than the free enzyme. Furthermore, the immobilized catalyst changes from a homogeneous to a heterogeneous form, which facilitates simple separation of the biocatalytic system from the reaction mixture. Because of this, it was possible to recycle the immobilized aminoacylase and retain 70% of its initial activity over at least five hydrolysis cycles. It should be also highlighted that proposed immobilization technique can be applied to other enzymes. This make it unique because enables preparation of novel, functional biocatalysts characterized with wide range of potential application.
References
Abdollahi, K., Yazdani, F., Panahi, R.: Covalent immobilization of tyrosinase onto cyanuric chloride crosslinked amine-functionalized superparamagnetic nanoparticles: synthesis and characterization of the recyclable nanobiocatalyst. Int. J. Biol. Macromol. 94, 396–405 (2017)
Ambrogio, M.W., Thomas, C.R., Zhao, Y.L., Zink, J.I., Stoddart, J.F.: Mechanized silica nanoparticles: a new frontier in theranostic nanomedicine. Acc. Chem. Res. 44, 903–913 (2011)
Antecka, K., Zdarta, J., Siwińska-Stefańska, K., Sztuk, G., Jankowska, E., Oleskowicz-Popiel, P., Jesionowski, T.: Synergistic degradation of dye wastewaters using binary or ternary oxide systems with immobilized laccase. Catalysts 8, 402–420 (2018)
Cai, C., Gao, Y., Liu, Y., Zhong, N., Liu, N.: Immobilization of Candida antarctica lipase B onto SBA-15 and their application in glycerolysis for diacylglycerols synthesis. Food Chem. 212, 205–212 (2016)
Cengiz, S., Cavas, L., Yurdakoc, K.: Bentonite and sepiolite as supporting media: immobilization of catalase. Appl. Clay Sci. 65–66, 114–120 (2012)
Chao, C., Liu, J., Wang, J., Zhang, Y., Zhang, B., Zhang, Y., Xiang, X., Chen, R.: Surface modification of halloysite nanotubes with dopamine for enzyme immobilization. Appl. Mater. Interfaces 5, 10559–10564 (2013)
Ciesielczyk, F., Goscianska, J., Zdarta, J., Jesionowski, T.: The development of zirconia/silica hybrids for the adsorption and controlled release of active pharmaceutical ingredients. Colloid Surf. A 545, 39–50 (2018)
Coutinho, T.C., Rojas, M.J., Tardioli, P.W., Paris, E.C., Farinas, C.S.: Nanoimmobilization of β-glucosidase onto hydroxyapatite. Int. J. Biol. Macromol. 119, 1042–1051 (2018)
Cowana, A., Fernandez-Lafuente, R.: Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb. Technol. 49, 326–346 (2011)
Del Angel-Lopez, D., Torres-Huerta, A.M., Dominguez-Crespo, M.A., Onofre-Bustamante, E.: Effect of ZrO2:SiO2 dispersion on the thermal stability, mechanical properties and corrosion behavior of hybrid coatings deposited on carbon steel. J. Alloys Compd. 615, S423–S432 (2015)
Das, R., Ranjan, R., Sinha, N., Kayastha, A.M.: Comparative characterization of peanut β-amylase immobilization onto graphene oxide and graphene oxide carbon nanotubes by solid-state NMR. J. Phys. Chem. C 122, 19259–19265 (2018)
Demkina, E.V., Shanenko, E.F., Nikolaev, Y.A., El’-Registan, G.I.: Model of the regulation of activity of immobilized enzymes (amylases) in soil. Microbiology 86, 231–240 (2017)
Forde, J., Vakurov, A., Gibson, T.D., Millner, P., Whelehan, M., Marison, I.W., O’Fagain, C.: Chemical modification and immobilisation of lipase B from Candida antarctica onto mesoporous silicates. J. Mol. Catal. B 66, 203–209 (2010)
Guncheva, M., Paunova, K., Dimitrov, M., Yancheva, D.: Stabilization of Candida rugosa lipase on nanosized zirconia-based materials. J. Mol. Catal. B 108, 43–50 (2014)
Haghighi, N., Hallaj, R., Salimi, A.: Immobilization of glucose oxidase onto a novel platform based on modified TiO2 and graphene oxide, direct electrochemistry, catalytic and photocatalytic activity. Mater. Sci. Eng. 73, 417–424 (2017)
Heater, B.S., Lee, M.M., Chan, M.K.: Direct production of a genetically-encoded immobilized biodiesel catalyst. Sci. Rep. 8, 12783–12793 (2018)
Homaei, A., Sariri, R., Vianello, F.: Enzyme immobilization: an update. J. Chem. Biol. 6, 185–205 (2013)
Jesionowski, T., Krysztafkiewicz, A.: Influence of silane coupling agents on surface properties of precipitated silicas. Appl. Surf. Sci. 172, 18–32 (2001)
Jesionowski, T., Zdarta, J., Krajewska, B.: Enzyme immobilization by adsorption: a review. Adsorption 20, 801–821 (2014)
Liang, J., Srinivasan, P.B., Blawert, C., Dietzel, W.: Comparison of electrochemical corrosion behaviour of MgO and ZrO2 coatings on AM50 magnesium alloy formed by plasma electrolytic oxidation. Corros. Sci. 51, 2483–2492 (2009)
Jin, Q., Li, X., Deng, C., Zhang, Q., Yi, D., Wang, X., Tang, Y., Wang, Y.: Silica nanowires with tunable hydrophobicity for lipase immobilization and biocatalytic membrane assembly. J. Colloid Interface Sci. 531, 555–563 (2018)
Klapiszewski, L., Królak, M., Jesionowski, T.: Silica synthesis by the sol-gel method and its use in the preparation of multifunctional biocomposites. Cent. Eur. J. Chem. 12, 173–184 (2014)
Kolodziejczak-Radzimska, A.: Functionalized Stober silica as a support in immobilization process of lipase from Candida rugosa. Physicochem. Probl. Miner. Process. 53, 878–892 (2017)
Kolodziejczak-Radzimska, A., Zdarta, J., Jesionowski, T.: Physicochemical and catalytic properties of acylase I from Aspergillus melleus immobilized on amino- and carbonyl-grafted Stober silica. Biotech. Progress 34, 767–777 (2018a)
Kolodziejczak-Rdzimska, A., Zdarta, J., Ciesielczyk, F., Jesionowski, T.: An organofunctionalized MgO·SiO2 hybrid support and its performance in the immobilization of lipase from Candida rugosa. Korean J. Chem. Eng. 35, 2220–2231 (2018b)
Li, J., Zhao, Z., Mo, T., Wang, L., Li, P.: Immobilization of aminoacylase on electrospun nanofibrous membrane for the resolution of dl-theanine. J. Mol. Catal. B 116, 24–28 (2015)
Masuda, Y., Kugimiya, S., Kato, K.: Improvement of thermal-stability of enzyme immobilized onto mesoporous zirconia. J. Asian Ceram. Soc. 2, 11–19 (2014)
Mateo, C., Palomo, J., Fernandez-Lorente, G.: Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 40, 1451–1463 (2007)
Mehde, A.A., Mehdi, W.A., Severgun, O., Cakar, S., Ozacar, M.: Lipase-based on starch material as a development matrix with magnetite cross-linked enzyme aggregates and its application. Int. J. Biol. Macromol. 120, 1533–1543 (2018)
Mohiuddin, M., Arbain, D., Islam, A.K.M.S., Rahman, M., Ahmad, M.S., Ahmad, M.N.: Covalent immobilization of α-glucosidase enzyme onto amine functionalized multi-walled carbon nanotubes. Curr. Nanosci. 5, 730–735 (2014)
Noori, N., Nikoorazm, M., Ghorbani-Choghamarani, A.: Oxo-vanadium immobilized on l-cysteine-modified MCM-41 as catalyst for the oxidation of sulfides and oxidative coupling of thiols. Microporous Mesoporous Mater. 234, 166–175 (2016)
Pang, S., Wu, Y., Zhang, X., Li, B., Ouyang, J., Ding, M.: Immobilization of laccase via adsorption onto bimodal mesoporous Zr-MOF. Process Biochem. 51, 229–239 (2016)
Piccinini, E., Bliem, C., Rozman, C.R., Battaglini, F., Azzaroni, O., Knoll, W.: Enzyme-polyelectrolyte multilayer assemblies on reduced graphene oxide field-effect transistors for biosensing applications. Biosens. Bioelectron. 92, 661–667 (2017)
Pogorilyi, R.P., Pylypchuk, I., Melnyk, I.V., Zub, Y.L., Seisenbaeva, G.A., Kessler, V.G.: Sol-gel derived adsorbents with enzymatic and complexonate functions for complex water remediation. Nanomaterials 7, 298–315 (2017)
Pylypchuk, I.V., Kessler, V.G., Seisenbaeva, G.A.: Simultaneous removal of acetaminophen, diclofenac, and Cd(II) by Trametes versicolor laccase immobilized on Fe3O4/SiO2-DTPA hybrid nanocomposites. ACS Sustain. Chem. Eng. 6, 9979–9989 (2018)
Rahulan, K.M., Vinitha, G., Stephen, D.L, Kanakam, C.C.: Synthesis and optical limiting effects in ZrO2 and ZrO2@SiO2 core shell nanostructures. Ceram. Int. 39, 5281–5286 (2013)
Santos, M., Lobo, I., Cruz, R.: Synthesis and characterization of novel ZrO2∙SiO2 mixed oxides. Mater. Res. 17, 700–707 (2014)
Shah, P., Sridevi, N., Prabhune, A., Ramaswamy, V.: Structural features of Penicillin acylase adsorption on APTES functionalized SBA-15. Microporous Mesoporous Mater. 116, 157–165 (2008)
Shibli, S.M.A., Chacko, F., Divya, C.: Al2O3–ZrO2 mixed oxide composite incorporated aluminium rich zinc coatings for high wear resistance. Corros. Sci. 52, 518–525 (2010)
Szewczuk-Karpisz, K., Wisniewska, M.: Lysozyme as a flocculant-inducing agent improving the silica removal from aqueous solutions - a turbidimetric study. J. Environ. Manage. 226, 187–193 (2018)
Tak, M., Gupta, V., Tomar, M.: A highly efficient urea detection using flower-like zinc oxide nanostructures. Mater. Sci. Eng., C 57, 38–48 (2017)
Vaizogullar, A., Balci, A., Ugurlu, M.: Synthesis of ZrO2 and ZrO2/SiO2 particles and photocatalytic degradation of methylene blue. Indian J. Chem. 54, 1434–1439 (2015)
Wisniewska, M., Ostolska, I., Szewczuk-Karpisz, K., Chibowski, S., Terpiłowski, K., Gunko, V.M., Zarko, V.I.: Investigation of the polyvinyl alcohol stabilization mechanism and adsorption properties on the surface of ternary mixed nanooxide AST 50 (Al2O3–SiO2–TiO2). J. Nanoparticle Res. 17, 1–14 (2015)
Wu, H., Liang, Y., Shi, J.: Enhanced stability of catalase covalently immobilized on functionalized titania submicrospheres. Mater. Sci. Eng. 33, 1438–1445 (2013)
Wu, F., Su, L., Yu, P., Mao, L.: Role of organic solvents in immobilizing fungus laccase on single-walled carbon nanotubes for improved current response in direct bioelectrocatalysis. J. Am. Chem. Soc. 139, 1565–1574 (2017)
Xiao, Q.G., Tao, X., Zhang, J.P., Chen, J.F.: Hollow silica nanotubes for immobilization of penicillin G acylase enzyme. J. Mol. Cat. B 42, 14–19 (2006)
Xiao, Y.F., Yao, Z., Wang, H.Q., Xiong, Q., Hu, G.M., Xu, H., Wei, P.: Enzymatic synthesis of S-bzl-γ-glutamyl-l-cysteine with γ-glutamyltranspeptidase immobilized onto ordered mesoporous TiO2. Chin. J. Chem. Eng. 10, 1175–1180 (2010)
Zdarta, J., Meyer, A.S., Jesionowski, T., Pinelo, M.: A general overview of support materials for enzyme immobilization: characteristics, properties, practical utility. Catalysts 8, 92–119 (2018)
Zdarta, J., Salek, K., Kolodziejczak-Radzimska, A., Siwinska-Stefanska, K., Szwarc-Rzepka, K., Norman, M., Klapiszewski, Ł., Bartczak, P., Kaczorek, E., Jesionowski, T.: Immobilization of Amano Lipase A onto Stöber silica surface: process characterization and kinetic studies. Open Chem. 13, 138–148 (2015)
Zhai, R., Zhang, B., Liu, L., Xie, Y., Zhang, H., Liu, J.: Immobilization of enzyme biocatalyst on natural halloysite nanotubes. Catal. Commun. 12, 259–263 (2010)
Zivkovic, L.T.I., Zivkovic, L.S., Babic, B.M., Kokunesoski, M.J., Jokic, B.M., Karadzic, I.M.: Immobilization of Candida rugosa lipase by adsorption onto biosafe meso/macroporous silica and zirconia. Biochem. Eng. J. 93, 73–83 (2015)
Zivkovic, L.T.I., Zivkovic, L.S., Beskoskic, V.P., Gopcevica, K.R., Jokic, B.M., Radosavljevice, D.S., Karadzica, I.M.: The Candida rugosa lipase adsorbed onto titania as nano biocatalyst with improved thermostability and reuse potential in aqueous and organic media. J. Mol. Catal. B 133, 533–542 (2016)
Acknowledgements
This work was supported by the Polish Ministry of Science and Higher Education (Grant No. 03/32/SBAD/0906).
Funding
Funding was provided by Politechnika Poznańska.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to S.I. ISSHAC10, but it reach the press at the time the special issue was published.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kołodziejczak-Radzimska, A., Ciesielczyk, F. & Jesionowski, T. A novel biocatalytic system obtained via immobilization of aminoacylase onto sol–gel derived ZrO2·SiO2 binary oxide material: physicochemical characteristic and catalytic activity study. Adsorption 25, 855–864 (2019). https://doi.org/10.1007/s10450-019-00085-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-019-00085-7