Abstract
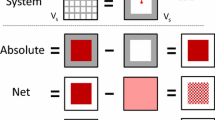
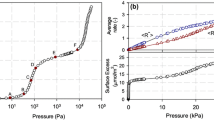
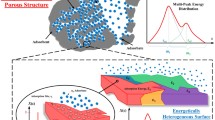
This paper addresses the long-standing problem of the so-called Gibbs dividing surface and the use of helium as a “non-adsorbing” gas for the determination of the “helium”-void volume and thence the Gibbs excess. Using helium is subject to some uncertainty because helium does adsorb (to call it a non-adsorbing gas is misleading) and it is able to access pore spaces that other larger adsorbates cannot. On the other hand, even helium atoms can not physically probe all the space described by the helium-void volume. To avoid these difficulties, we suggest an alternative to the formulation of the Gibbs dividing surface and the definition of the excess amount. We illustrate this with the two common tools to study adsorption—the volumetric and gravimetric techniques, and justify our new analysis with a computer simulation of a number of model adsorption systems. Furthermore, we also show that by using the correct accessible volume and inaccessible volume the excess amount obtained from a volumetric experiment is exactly the same as that obtained from a gravimetric experiment.
Similar content being viewed by others
References
Benson, G.C., Yun, K.S.: In: Flood, E.A. (ed.) The Solid-Gas Interface, pp. 203–264, New York (1967)
Do, D.D., Do, H.D.: Appropriate volumes for adsorption isotherm studies: the absolute void volume, accessible pore volume and enclosing particle volume. J. Colloid Interface Sci. 316(2), 317–330 (2007)
Do, D.D., Nicholson, D., Do, H.D.: On the Henry constant and isosteric heat at zero loading in gas phase adsorption. J. Colloid Interface Sci. 324(1–2), 15–24 (2008a)
Do, D.D., et al.: Henry constant and isosteric heat at zero-loading for gas adsorption in carbon nanotubes. Phys. Chem. Chem. Phys. 10, 7293–7303 (2008b)
Do, D.D., et al.: On the existence of negative excess isotherms for argon adsorption on graphite surfaces and in graphitic pores under supercritical conditions at pressures up to 10,000 atm. Langmuir 26(7), 4796–4806 (2010)
Do, D., Herrera, L., Nicholson, D.: A method for the determination of accessible surface area, pore volume, pore size and its volume distribution for homogeneous pores of different shapes. Adsorption 17(2), 325–335 (2011)
Gumma, S., Talu, O.: Gibbs dividing surface and helium adsorption. Adsorption 9(1), 17–28 (2003)
Gumma, S., Talu, O.: Net adsorption: a thermodynamic framework for supercritical gas adsorption and storage in porous solids. Langmuir 26(22), 17013–17023 (2010)
Herrera, L., Do, D.D., Nicholson, D.: A Monte Carlo integration method to determine accessible volume, accessible surface area and its fractal dimension. J. Colloid Interface Sci. 348(2), 529–536 (2010a)
Herrera, L., et al.: A novel and consistent method (TriPOD) to characterize an arbitrary porous solid for its accessible volume, accessible geometrical surface area and accessible pore size. Adsorption 17(1), 55–68 (2010b)
Herrera, L.F., et al.: Monte Carlo optimization scheme to determine the physical properties of porous and nonporous solids. Langmuir 26(19), 15278–15288 (2010c)
Herrera, L.F., et al.: Novel method to determine accessible volume, area, and pore size distribution of activated carbon. Industrial & Engineering Chemistry Research (2011)
Myers, A.L., Monson, P.A.: Adsorption in porous materials at high pressure: theory and experiment. Langmuir 18(26), 10261–10273 (2001)
Myers, A.L., Talu, O.: Molecular simulation of adsorption: Gibbs dividing surface and comparison with experiment. AIChE 47(5), 1160 (2001)
Neimark, A.V., Ravikovitch, P.I.: Calibration of pore volume in adsorption experiments and theoretical models. Langmuir 13(19), 5148–5160 (1997)
Peterson, B.K., Gubbins, K.E.: Phase transitions in a cylindrical pore—grand canonical Monte Carlo, mean field theory and the Kelvin equation. Mol. Phys. 62(1), 215–226 (1987)
Rowlinson, J.S., Widom, B.: Molecular Theory of Capillarity. Clarendon Press, Oxford (1982)
Sircar, S.: R&D note: data representation for binary and multicomponent gas adsorption equilibria. Adsorption 2(4), 327–330 (1996)
Sircar, S.: Gibbsian surface excess for gas adsorption revisited. Ind. Eng. Chem. Res. 38(10), 3670–3682 (1999)
Sircar, S.: Role of helium void measurement in estimation of Gibbsian surface excess. In: Fundamentals of Adsorption, vol. 7, Chiba, Japan (2001a)
Sircar, S.: Measurement of Gibbsian surface excess. AIChE J. 47(5), 1169–1176 (2001b)
Talu, O.: Needs, status, techniques and problems with binary gas adsorption experiments. Adv. Colloid Interface Sci. 76–77 227–269 (1998)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrera, L., Fan, C., Do , D.D. et al. A revisit to the Gibbs dividing surfaces and helium adsorption. Adsorption 17, 955–965 (2011). https://doi.org/10.1007/s10450-011-9374-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-011-9374-y