Abstract
von Willebrand Factor is a mechano-sensitive protein circulating in blood that mediates platelet adhesion to subendothelial collagen and platelet aggregation at high shear rates. Its hemostatic function and thrombogenic effect, as well as susceptibility to enzymatic cleavage, are regulated by a conformational change from a collapsed globular state to a stretched state. Therefore, it is essential to account for the conformation of the vWF multimers when modeling vWF-mediated thrombosis or vWF degradation. We introduce a continuum model of vWF unfolding that is developed within the framework of our multi-constituent model of platelet-mediated thrombosis. The model considers two interconvertible vWF species corresponding to the collapsed and stretched conformational states. vWF unfolding takes place via two regimes: tumbling in simple shear and strong unfolding in flows with dominant extensional component. These two regimes were demonstrated in a Couette flow between parallel plates and an extensional flow in a cross-slot geometry. The vWF unfolding model was then verified in several microfluidic systems designed for inducing high-shear vWF-mediated thrombosis and screening for von Willebrand Disease. The model predicted high concentration of stretched vWF in key regions where occlusive thrombosis was observed experimentally. Strong unfolding caused by the extensional flow was limited to the center axis or middle plane of the channels, whereas vWF unfolding near the channel walls relied upon the shear tumbling mechanism. The continuum model of vWF unfolding presented in this work can be employed in numerical simulations of vWF-mediated thrombosis or vWF degradation in complex geometries. However, extending the model to 3-D arbitrary flows and turbulent flows will pose considerable challenges.

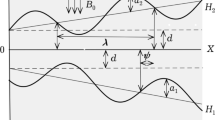
Adapted from Woo and Shaqfeh,72 2003, with the permission of AIP Publishing.







Similar content being viewed by others
References
Alexander-Katz, A., and R. R. Netz. Surface-enhanced unfolding of collapsed polymers in shear flow. EPL. 80:18001, 2007.
Alexander-Katz, A., and R. R. Netz. Dynamics and instabilities of collapsed polymers in shear flow. Macromolecules. 41:3363–3374, 2008.
Alexander-Katz, A., M. F. Schneider, S. W. Schneider, A. Wixforth, and R. R. Netz. Shear-flow-induced unfolding of polymeric globules. Phys. Rev. Lett. 97:1–4, 2006.
Babcock, H. P., R. E. Teixeira, J. S. Hur, E. S. G. Shaqfeh, and S. Chu. Visualization of molecular fluctuations near the critical point of the coil−stretch transition in polymer elongation. Macromolecules. 36:4544–4548, 2003.
Bark, D. L., A. N. Para, and D. N. Ku. Correlation of thrombosis growth rate to pathological wall shear rate during platelet accumulation. Biotechnol. Bioeng. 109:2642–2650, 2012.
Bartoli, C. R., D. J. Restle, D. M. Zhang, M. A. Acker, and P. Atluri. Pathologic von Willebrand factor degradation with a left ventricular assist device occurs via two distinct mechanisms: Mechanical demolition and enzymatic cleavage. J. Thorac. Cardiovasc. Surg. 149:281–289, 2015.
Baumann Kreuziger, L., M. S. Slaughter, K. Sundareswaran, and A. E. Mast. Clinical relevance of histopathologic analysis of heartmate II thrombi. ASAIO J. 2018. https://doi.org/10.1097/MAT.0000000000000759.
Blackburn, H. M., N. N. Mansour, and B. J. Cantwell. Topology of fine-scale motions in turbulent channel flow. J. Fluid Mech. 310:269–292, 1996.
Bortot, M., K. Ashworth, A. Sharifi, F. Walker, N. C. Crawford, K. B. Neeves, D. Bark, and J. Di Paola. Turbulent flow promotes cleavage of VWF (von Willebrand factor) by ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type-1 motif, member 13). Arterioscler. Thromb. Vasc. Biol. 39:1831–1842, 2019.
Casa, L. D. C., D. H. Deaton, and D. N. Ku. Role of high shear rate in thrombosis. J. Vasc. Surg. 61:1068–1080, 2015.
Chong, M. S., A. E. Perry, and B. J. Cantwell. A general classification of three-dimensional flow fields. Phys. Fluids A. 2:765–777, 1990.
Chorin, A. J. Vorticity and Turbulence. New York: Springer, 1994.
Coghill, P. A., S. Kanchi, Z. J. Azartash-Namin, J. W. Long, and T. A. Snyder. Benchtop von Willebrand factor testing. ASAIO J. 2018. https://doi.org/10.1097/MAT.0000000000000849.
Danish, M., and C. Meneveau. Multiscale analysis of the invariants of the velocity gradient tensor in isotropic turbulence. Phys. Rev. Fluids. 3:1–22, 2018.
De Gennes, P. G. Coil-stretch transition of dilute flexible polymers under ultrahigh velocity gradients. J. Chem. Phys. 5030:5030–5042, 1974.
Dong, C., S. Kania, M. Morabito, X. F. Zhang, W. Im, A. Oztekin, X. Cheng, and E. B. Webb. A mechano-reactive coarse-grained model of the blood-clotting agent von Willebrand factor. J. Chem. Phys. 151:124905, 2019.
Dunlap, P. N., and L. G. Leal. Dilute polystyrene solutions in extensional flows: Birefringence and flow modification. J. Nonnewton. Fluid Mech. 23:5–48, 1987.
Faghih, M. M., and M. K. Sharp. Evaluation of energy dissipation rate as a predictor of mechanical blood damage. Artif. Organs. 43:666–676, 2019.
Favaloro, E. J. Clinical utility of the PFA-100. Semin. Thromb. Hemost. 34:709–733, 2008.
Fu, H., Y. Jiang, D. Yang, F. Scheiflinger, W. P. Wong, and T. A. Springer. Flow-induced elongation of von Willebrand factor precedes tension-dependent activation. Nat. Commun. 8:1–12, 2017.
Fuller, G. G., and L. G. Leal. Flow birefringence of dilute polymer solutions in two-dimensional flows. Rheol. Acta. 19:580–600, 1980.
Harrison, P., M. Robinson, R. Liesner, K. Khair, H. Cohen, I. Mackie, and S. Machin. The PFA-100®: a potential rapid screening tool for the assessment of platelet dysfunction. Clin. Lab. Haematol. 24:225–232, 2002.
Haward, S. J. Microfluidic extensional rheometry using stagnation point flow. Biomicrofluidics. 10:043401, 2016.
Hund, S. J., J. F. Antaki, and M. Massoudi. On the representation of turbulent stresses for computing blood damage. Int. J. Eng. Sci. 48:1325–1331, 2010.
Hur, J. S., E. S. G. Shaqfeh, H. P. Babcock, and S. Chu. Dynamics and configurational fluctuations of single DNA molecules in linear mixed flows. Phys. Rev. 66:3–6, 2002.
Hur, J. S., E. S. G. Shaqfeh, H. P. Babcock, D. E. Smith, and S. Chu. Dynamics of dilute and semidilute DNA solutions in the start-up of shear flow. J. Rheol. 45:421–450, 2001.
Jendrejack, R. M., J. J. De Pablo, and M. D. Graham. Stochastic simulations of DNA in flow: Dynamics and the effects of hydrodynamic interactions. J. Chem. Phys. 116:7752–7759, 2002.
Kania, S., A. Oztekin, X. Cheng, X. F. Zhang, and E. Webb. Predicting pathological von Willebrand factor unraveling in elongational flow. Biophys. J. 2021. https://doi.org/10.1016/j.bpj.2021.03.008.
Kim, D., C. Bresette, Z. Liu, and D. N. Ku. Occlusive thrombosis in arteries. APL Bioeng. 3:041502, 2019.
Konnigk, L., B. Torner, M. Bruschewski, S. Grundmann, and F. H. Wurm. Equivalent scalar stress formulation taking into account non-resolved turbulent scales. Cardiovasc. Eng. Technol. 2021. https://doi.org/10.1007/s13239-021-00526-x.
Kragh, T., M. Napoleone, M. A. Fallah, H. Gritsch, M. F. Schneider, and A. J. Reininger. High shear dependent von willebrand factor self-assembly fostered by platelet interaction and controlled by ADAMTS13. Thromb. Res. 133:1079–1087, 2014.
Kundu, S. K., E. J. Heilmann, R. Sio, C. Garcia, R. M. Davidson, and R. A. Ostgaard. Description of an in vitro platelet function analyzer - PFA-100®. Semin. Thromb. Hemost. 21:106–112, 1995.
Larson, R. G. The rheology of dilute solutions of flexible polymers: Progress and problems. J. Rheol. 49:1–70, 2005.
Lippok, S., T. Obser, J. P. Müller, V. K. Stierle, M. Benoit, U. Budde, R. Schneppenheim, and J. O. Rädler. Exponential size distribution of von Willebrand factor. Biophys. J. 105:1208–1216, 2013.
Lippok, S., M. Radtke, T. Obser, L. Kleemeier, R. Schneppenheim, U. Budde, R. R. Netz, and J. O. Rädler. Shear-induced unfolding and enzymatic cleavage of full-length VWF multimers. Biophys. J. 110:545–554, 2016.
Liu, Z. L., D. N. Ku, and C. K. Aidun. Mechanobiology of shear-induced platelet aggregation leading to occlusive arterial thrombosis: A multiscale in silico analysis. J. Biomech. 120:110349, 2021.
Liu, Z., Y. Zhu, J. R. Clausen, J. B. Lechman, R. R. Rao, and C. K. Aidun. Multiscale method based on coupled lattice-Boltzmann and Langevin-dynamics for direct simulation of nanoscale particle/polymer suspensions in complex flows. Int. J. Numer. Methods Fluids. 91:228–246, 2019.
Lumley, J. L. Drag reduction by additives. Annu. Rev. Fluid Mech. 1:367–384, 1969.
Nascimbene, A., S. Neelamegham, O. H. Frazier, J. L. Moake, and J.-F. Dong. Acquired von Willebrand syndrome associated with left ventricular assist device. Blood. 127:3133–3141, 2016.
Nesbitt, W. S., E. Westein, F. J. Tovar-Lopez, E. Tolouei, A. Mitchell, J. Fu, J. Carberry, A. Fouras, and S. P. Jackson. A shear gradient–dependent platelet aggregation mechanism drives thrombus formation. Nat. Med. 15:665–673, 2009.
Ng, R.C.-Y., and L. G. Leal. Concentration effects on birefringence and flow modification of semidilute polymer solutions in extensional flows. J. Rheol. 37:443–468, 1993.
Ouyang, W., W. Wei, X. Cheng, X. F. Zhang, E. B. Webb, and A. Oztekin. Flow-induced conformational change of von Willebrand Factor multimer: Results from a molecular mechanics informed model. J. Nonnewton. Fluid Mech. 217:58–67, 2015.
Ozturk, M., E. A. O’Rear, and D. V. Papavassiliou. Hemolysis related to turbulent eddy size distributions using comparisons of experiments to computations. Artif. Organs. 39:E227–E239, 2015.
Para, A. N., and D. N. Ku. A low-volume, single pass in-vitro system of high shear thrombosis in a stenosis. Thromb. Res. 131:418–424, 2013.
Perkins, T. T. Single polymer dynamics in an elongational flow. Science. 276:2016–2021, 1997.
Peterson, D. M., N. A. Stathopoulos, T. D. Giorgio, J. D. Hellums, and J. L. Moake. Shear-induced platelet aggregation requires von Willebrand factor and platelet membrane glycoproteins Ib and IIb-IIIa. Blood. 69:625–628, 1987.
Proudfoot, A. G., S. J. Davidson, and M. Strueber. von Willebrand factor disruption and continuous-flow circulatory devices. J. Heart Lung Transpl. 36:1155–1163, 2017.
Rauch, A., S. Susen, and B. Zieger. Acquired von Willebrand syndrome in patients with ventricular assist device. Front. Med. 6:1–9, 2019.
Ruggeri, Z. M. Platelet adhesion under flow. Microcirculation. 16:58–83, 2009.
Ruggeri, Z. M., J. N. Orje, R. Habermann, A. B. Federici, and A. J. Reininger. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 108:1903–1910, 2006.
Savage, B., F. Almus-Jacobs, and Z. M. Ruggeri. Specific synergy of multiple substrate–receptor interactions in platelet thrombus formation under flow. Cell. 94:657–666, 1998.
Savage, B., E. Saldívar, and Z. M. Ruggeri. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 84:289–297, 1996.
Schneider, S. W., S. Nuschele, A. Wixforth, C. Gorzelanny, A. Alexander-Katz, R. R. Netz, and M. F. Schneider. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc. Natl. Acad. Sci. 104:7899–7903, 2007.
Schroeder, C. M., H. P. Babcock, E. S. G. Shaqfeh, and S. Chu. Observation of polymer conformation hysteresis in extensional flow. Science. 301:1515–1519, 2003.
Schroeder, C. M., R. E. Teixeira, E. S. G. Shaqfeh, and S. Chu. Characteristic periodic motion of polymers in shear flow. Phys. Rev. Lett. 95:1–4, 2005.
Shankaran, H., and S. Neelamegham. Hydrodynamic forces applied on intercellular bonds, soluble molecules, and cell-surface receptors. Biophys. J. 86:576–588, 2004.
Shaqfeh, E. S. G. The dynamics of single-molecule DNA in flow. J. Nonnewton. Fluid Mech. 130:1–28, 2005.
Sharifi, A., and D. Bark. Mechanical forces impacting cleavage of Von Willebrand factor in laminar and turbulent blood flow. Fluids. 6:67, 2021.
Siemens Healthineers AG. PFA-100 System. 2021. https://www.siemens-healthineers.com/en-us/hemostasis/systems/pfa-100
Sing, C. E., and A. Alexander-Katz. Elongational flow induces the unfolding of von willebrand factor at physiological flow rates. Biophys. J. 98:L35–L37, 2010.
Sing, C. E., and A. Alexander-Katz. Globule−stretch transitions of collapsed polymers in elongational flow fields. Macromolecules. 43:3532–3541, 2010.
Sing, C. E., and A. Alexander-Katz. Dynamics of collapsed polymers under the simultaneous influence of elongational and shear flows. J. Chem. Phys. 135:014902, 2011.
Smith, D. E. Single-polymer dynamics in steady shear flow. Science. 283:1724–1727, 1999.
Somani, S., E. S. G. Shaqfeh, and J. R. Prakash. Effect of solvent quality on the coil−stretch transition. Macromolecules. 43:10679–10691, 2010.
Tadmor, E. B., R. E. Miller, and R. S. Elliott. Continuum Mechanics and Thermodynamics. Cambridge: Cambridge University Press, 2011.
Tennekes, H., and J. L. Lumley. A First Course in Turbulence. Cambridge: The MIT Press, 1972.
Terrapon, V. E., Y. Dubief, P. Moin, E. S. G. Shaqfeh, and S. K. Lele. Simulated polymer stretch in a turbulent flow using Brownian dynamics. J. Fluid Mech. 504:61–71, 2004.
Tovar-Lopez, F. J., G. Rosengarten, E. Westein, K. Khoshmanesh, S. P. Jackson, A. Mitchell, and W. S. Nesbitt. A microfluidics device to monitor platelet aggregation dynamics in response to strain rate micro-gradients in flowing blood. Lab Chip. 10:291–302, 2010.
Vincentelli, A., S. Susen, T. Le Tourneau, I. Six, O. Fabre, F. Juthier, A. Bauters, C. Decoene, J. Goudemand, A. Prat, and B. Jude. Acquired von Willebrand syndrome in aortic stenosis. N. Engl. J. Med. 349:343–349, 2003.
von Springer, T. A. Willebrand factor, Jedi knight of the bloodstream. Blood. 124:1412–1425, 2014.
Westein, E., A. D. van der Meer, M. J. E. Kuijpers, J.-P. Frimat, A. van den Berg, and J. W. M. Heemskerk. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc. Natl. Acad. Sci. 110:1357–1362, 2013.
Woo, N. J., and E. S. G. Shaqfeh. The configurational phase transitions of flexible polymers in planar mixed flows near simple shear. J. Chem. Phys. 119:2908–2914, 2003.
Wu, P., Q. Gao, and P. L. Hsu. On the representation of effective stress for computing hemolysis. Biomech. Model. Mechanobiol. 18:665–679, 2019.
Wu, W.-T., M. A. Jamiolkowski, W. R. Wagner, N. Aubry, M. Massoudi, and J. F. Antaki. Multi-constituent simulation of thrombus deposition. Sci. Rep. 7:42720, 2017.
Wu, W. T., F. Yang, J. Wu, N. Aubry, M. Massoudi, and J. F. Antaki. High fidelity computational simulation of thrombus formation in Thoratec HeartMate II continuous flow ventricular assist device. Sci. Rep. 6:1–11, 2016.
Zhang, X., K. Halvorsen, C.-Z. Zhang, W. P. Wong, and T. A. Springer. Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science. 324:1330–1334, 2009.
Zhussupbekov, M., W.-T. Wu, M. A. Jamiolkowski, M. Massoudi, and J. F. Antaki. Influence of shear rate and surface chemistry on thrombus formation in micro-crevice. J. Biomech. 2021. https://doi.org/10.1016/j.jbiomech.2021.110397.
Acknowledgments
Author Wei-Tao Wu thanks the support of the Grant NSFC 11802135. This work was supported by the National Institute of Health Grant R01HL089456. Authors are grateful to Dr. Mahdi Esmaily Moghadam (Sibley School of Mechanical and Aerospace Engineering, Cornell University) for valuable discussions on turbulent flows.
Conflict of interest
Authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhussupbekov, M., Méndez Rojano, R., Wu, WT. et al. A Continuum Model for the Unfolding of von Willebrand Factor. Ann Biomed Eng 49, 2646–2658 (2021). https://doi.org/10.1007/s10439-021-02845-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02845-5