Abstract
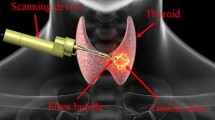
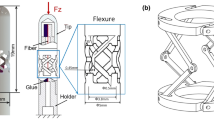
Optical biopsy methods, such as probe-based endomicroscopy, can be used to identify early-stage gastric cancer in vivo. However, it is difficult to scan a large area of the gastric mucosa for mosaicking during endoscopy. In this work, we propose a miniaturised flexible instrument based on contact-aided compliant mechanisms and fibre Bragg grating (FBG) sensing for intraoperative gastric endomicroscopy. The instrument has a compact design with an outer diameter of 2.7 mm, incorporating a central channel with a diameter of 1.9 mm for the endomicroscopic probe to pass through. Experimental results show that the instrument can achieve raster trajectory scanning over a large tissue surface with a positioning accuracy of 0.5 mm. The tip force sensor provides a 4.6 mN resolution for the axial force and 2.8 mN for transverse forces. Validation with random samples shows that the force sensor can provide consistent and accurate three-axis force detection. Endomicroscopic imaging experiments were conducted, and the flexible instrument performed no gap scanning (mosaicking area more than 3 mm2) and contact force monitoring during scanning, demonstrating the potential of the system in clinical applications.








Similar content being viewed by others
References
Balicki, M., A. Uneri, I. Iordachita, J. Handa, P. Gehlbach, and R. Taylor. Micro-force sensing in robot assisted membrane peeling for vitreoretinal surgery. In: International Conference on Medical Image Computing and Computer-Assisted Intervention, 2010, pp. 303–310.
Bray, F., J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre, and A. Jemal. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 68(6):394–424, 2018.
Burgner-Kahrs, J., D. C. Rucker, and H. Choset. Continuum robots for medical applications: a survey. IEEE Trans. Robot. 31(6):1261–1280, 2015.
Chekotu, J. C., R. Groarke, K. O’Toole, and D. Brabazon. Advances in selective laser melting of nitinol shape memory alloy part production. Materials. 12(5):809, 2019.
Cutsem, E. V., X. Sagaert, B. Topal, K. Haustermans, and H. Prenen. Gastric cancer. Lancet. 388(10060):2654–2664, 2016.
Erden, M. S., B. Rosa, N. Boularot, B. Gayet, G. Morel, and J. Szewczyk. Conic-Spiraleur: a miniature distal scanner for confocal microlaparoscope. IEEE/ASME Trans. Mechatron. 19(6):1786–1798, 2014.
Giataganas, P., M. Hughes, C. J. Payne, P. Wisanuvej, B. Temelkuran, and G. Z. Yang. Intraoperative robotic-assisted large-area high-speed microscopic imaging and intervention. IEEE Trans. Biomed. Eng. 66(1):208–216, 2019.
Gong, L., J. Zheng, Z. Ping, Y. Wang, and S. Zuo. Robust mosaicing of endomicroscopic videos via context-weighted correlation ratio. IEEE Trans. Biomed. Eng. 2020. https://doi.org/10.1109/TBME.2020.3007768.
He, X., J. Handa, T., P. Gehlbach, R. Taylor, and I. Iordachita. A Submillimetric 3-DOF force sensing instrument with integrated fiber bragg grating for retinal microsurgery. IEEE Trans. Biomed. Eng. 61(2):522–534, 2014.
Hong, W., L. Xie, J. Liu, Y. Sun, K. Li, and H. Wang. Development of a novel continuum robotic system for maxillary sinus surgery. IEEE/ASME Trans. Mechatron. 23(3):1226–1237, 2018.
Kitabatake, S., Y. Niwa, R. Miyahara, A. Ohashi, T. Matsuura, Y. Iguchi, Y. Shimoyama, T. Nagasaka, O. Maeda, T. Ando, N. Ohmiya, A. Itoh, Y. Hirooka, and H. Goto. Confocal endomicroscopy for the diagnosis of gastric cancer in vivo. Endoscopy. 38(11):1110–1114, 2006.
Kutzer, M. D. M., S. M. Segreti, C. Y. Brown, R. H. Taylor, M. Armand and S. C. Mears. Design of a new cable-driven manipulator with a large open lumen: Preliminary applications in the minimally-invasive removal of osteolysis. In: IEEE International Conference on Robotics and Automation, 2011, pp. 2913–2920.
Latt, W. T., R. C. Newton, M. Visentini-Scarzanella, C. J. Payne, D. P. Noonan, J. Shang, and G. Z. Yang. A hand-held instrument to maintain steady tissue contact during probe-based confocal laser endomicroscopy. IEEE Trans. Biomed. Eng. 58(9):2694–2703, 2011.
Liu, J., B. Hall, M. Frecker, and E. W. Reutzel. Compliant articulation structure using superelastic NiTiNOL. Smart Mater. Struct. 22(9):2013.
Luo, M., and L. Li. Clinical utility of miniprobe endoscopic ultrasonography for prediction of invasion depth of early gastric cancer: a meta-analysis of diagnostic test from PRISMA guideline. Medicine. 98(6):2019.
Miyashita, K., T. O. Vrielink, and G. Mylonas. A cable-driven parallel manipulator with force sensing capabilities for high-accuracy tissue endomicroscopy. Int. J. Comput. Assist. Radiol. 13(5):659–669, 2018.
Newton, R. C., S. V. Kemp, G. Z. Yang, D. Ellson, A. Darzi, and P. Shah. Imaging parenchymal lung diseases with confocal endomicroscopy. Respir. Med. 106(1):127–137, 2012.
Schmitz, A., Shen. T, P. Berthet-Rayne, and G. Z. Yang. A rolling-tip flexible instrument for minimally invasive surgery. In: IEEE International Conference on Robotics and Automation, 2019, pp. 379–385.
Sonn, G. A., S.-N. E. Jones, T. V. Tarin, C. B. Du, K. E. Mach, K. C. Jensen, and J. C. Liao. Optical biopsy of human bladder neoplasia with in vivo confocal laser endomicroscopy. J. Urol. 182(4):1299–1305, 2009.
Takahisa, T., I. Okumura, H. Kose, K. Takagiet, and N. Hata. Tendon-driven continuum robot for neuroendoscopy: validation of extended kinematic mapping for hysteresis operation. Int. J. Comput. Assist. Radiol. 11(4):589–602, 2016.
Thompson, C. C., M. Ryou, N. J. Soper, E. S. Hungess, R. I. Rothstein, and L. L. Swanstrom. Evaluation of a manually driven, multitasking platform for complex endoluminal and natural orifice transluminal endoscopic surgery applications. Gastrointestinal Endoscopy. 70(1):121–125, 2009.
Vercauteren, T., A. Meining, F. Lacombe, and A. Perchant. Real time autonomous video image registration for endomicroscopy: fighting the compromises. Biomed. Opt. 6861:68610C, 2008.
Wallace, M. B., G. Y. Lauwers, Y. Chen, E. Dekker, P. Fockens, P. Sharma, and A. Meining. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy. 43(10):882–891, 2011.
Wang, H., S. Wang, J. Li, and S. Zuo. Robotic scanning device for intraoperative thyroid gland endomicroscopy. Ann. Biomed. Eng. 46(4):543–554, 2018.
Wisanuvej, P., P. Giataganas, K. Leibrandt, J. Liu, M. Hughes, and G. Z. Yang. Three-dimensional robotic-assisted endomicroscopy with a force adaptive robotic arm. In: IEEE International Conference on Robotics and Automation, 2017, pp. 2379–2384.
York, P. A., P. J. Swaney, H. B. Gilbert, and R. J. Webster III. A Wrist for Needle-Sized Surgical Robots. In: IEEE International Conference on Robotics and Automation, 2015, pp. 2964–2970.
Zorn, L., F. Nageotte, P. Zanne, A. Legner, B. Dallemagne, J. Marescaux, and M. Mathelin. A novel telemanipulated robotic assistant for surgical endoscopy: preclinical application to esd. IEEE Trans. Biomed. Eng. 65(4):797–808, 2018.
Zuo, S., M. Hughes, C. Seneci, T. P. Chang, and G. Z. Yang. Towards intraoperative breast endomicroscopy with a novel surface scanning device. IEEE Trans. Biomed. Eng. 62(12):2941–2952, 2015.
Acknowledgments
This work was supported in part by National Key R&D Program of China under Grant 2019YFB1311501 and in part by National Natural Science Foundation of China under Grant 61773280.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ka-Wai Kwok oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ping, Z., Zhang, T., Gong, L. et al. Miniature Flexible Instrument with Fibre Bragg Grating-Based Triaxial Force Sensing for Intraoperative Gastric Endomicroscopy. Ann Biomed Eng 49, 2323–2336 (2021). https://doi.org/10.1007/s10439-021-02781-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02781-4