Abstract
The optimal use of erythropoiesis stimulating agents to treat anemia of end-stage renal disease remains difficult due to reported associations with adverse events. A patient’s hemoglobin response to these agents cannot be accurately described using population-level models due to many individual factors including chronic inflammation, red blood cell lifespan, and acute blood loss. As a consequence, it is generally understood that current one-size-fits-all anemia management protocols result in suboptimal outcomes. In this paper, we report on our collaboration with the medical community in designing anemia management protocols. In clinical implementation, these new dosing protocols have led to improved outcomes due to their use of control-relevant modelling, model parameter identification, and principles of feedback control. This is an example of medical professionals and control engineers working together to positively affect the performance of anemia management protocols in end-stage renal disease.








Similar content being viewed by others
References
Barbieri, C., M. Molina, P. Ponce, M. Tothova, I. Cattinelli, J. Titapiccolo, F. Mari, V. Amato, F. Leipold, W. Wehmeyer, S. Stuard, A. Stopper, and B. Canaud. An international observational study suggests that artificial intelligence for clinical decision support optimizes anemia management in hemodialysis patients. Kidney Int. 90(2):422–429, 2016.
Bellazzi, R. Drug delivery optimization through Bayesian networks: an application to erythropoietin therapy in uremic anemia. Comput. Biomed. Res. 26(3):274–293, 1993.
Berns, J., S. H. Elzein, R. I. Lynn, S. Fishbane, I. S. Meisels, and P. B. Deoreo. Hemoglobin variability in epoetin-treated hemodialysis patients. Kidney Int. 64:1514–1521, 2003.
Biro A., S. Blumberg, R. Rachmilevich, Y. Chait, M. J. Germain, R. Cernes, Z. Barnea, and Z. Katzir. Engineering-based individualized anemia management in hemodialysis patients. American Society of Nephrology Kidney Week, New Orleans, LA, Oct 31–Nov 5, 2017.
Borghesani C, Y. Chait, and O. Yaniv. The Quantitative Feedback Theory (QFT) Frequency Domain Control Design Toolbox, 2003. http://128.119.85.95/cbs/software.html.
Brier, M. E., A. E. Gaweda, A. Dailey, G. R. Aronoff, and A. A. Jacobs. Randomized trial of model predictive control for improved anemia management. Clin. J. Am. Soc. Nephrol. 5(5):814–820, 2010.
Chait Y., M. J. Germain, C. V. Hollot, J. Horowtiz, and W. C. Vogt. Methods and Devices for Determining Optimal Agent Dosages, United States patent US 10,049,18782, 2018.
Chait, Y., J. Horowitz, B. Nichols, R. P. Shrestha, C. V. Hollot, and M. J. Germain. Control-relevant erythropoiesis modeling in end-stage renal disease. IEEE Trans. Biomed. Eng. 61(3):658–664, 2014.
Chait Y., J. Slater, B. H. Nathanson, and M. J. Germain. The pursuit of a more physiologic iron and ESA treatment: results from a Triferic and individualized web-based ESA protocol QA project. American Society of Nephrology Kidney Week, San Diego, CA Oct 23-28, 2018.
Chalhoub, E., S. Frinak, G. Zasuwa, M. D. Faber, E. Peterson, A. Besarab, and J. Yee. De novo once-monthly darbepoetin alpha treatment for the anemia of chronic kidney disease using a computerized algorithmic approach. Clin. Nephrol. 76(1):1–8, 2011.
De Meester, J., B. Maes, A. De Vriese, B. De Moor, J. Donck, M. Helbert, B. Bammens, and S. Jamar. Fluctuations of haemoglobinaemia in chronic haemodialysis patients. Acta Clinica Belgica. 66–2:123–128, 2011.
Fishbane, S., and J. S. Berns. Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int. 68:1337–1343, 2005.
Gabutti, L., N. Lötscher, J. Bianda, C. Marone, G. Mombelli, and M. Burnier. Would artificial neural networks implemented in clinical wards help nephrologists in predicting epoetin responsiveness? BMC Nephrol. 8:7–13, 2006.
Gaweda, A. E., G. R. Aronoff, A. A. Jacobs, S. N. Rai, and M. E. Brier. Individualized anemia management reduces hemoglobin variability in hemodialysis patients. J. Am. Soc. Nephrol. 25(1):159–166, 2014.
Gaweda, A. E., A. A. Jacobs, G. R. Aronoff, and M. E. Brier. Model predictive control of erythropoietin administration in the anemia of ESRD. Am. J. Kidney Dis. 51(1):71–79, 2008.
Gaweda, A. E., M. A. Michael, Y. Chait, J. Horowitz, M. J. Germain, C. V. Hollot, and M. E. Brier. Anemia Management: To Hold or not to Hold. Atlanta: American Society of Nephrology Kidney Week, 2013.
Gaweda, A. E., M. K. Muezzinoglu, G. R. Aronoff, A. A. Jacobs, J. M. Zurada, and B. E. Brier. Individualization of pharmacological anemia management using reinforcement learning. Neural Netw. 18(5–6):826–834, 2005.
Germain, M. J., C. V. Hollot, J. Horowitz, R. P. Shrestha, and Y. Chait. A New Measure of Patient Responsiveness for Improving Anemia Management Protocols. San Diego: American Society of Nephrology Kidney Week, pp. 1–4, 2012.
Guyton, A. C., and J. E. Hall. Guyton and Hall Textbook of Medical Physiology (11th ed.). Philadelphia: Elsevier, p. 1114, 2006.
Ho, H. R., M. G. Germain, J. Garb, S. Picard, M. K. Mackie, C. Bartlett, and E. J. Will. Use of 12×/month haemoglobin monitoring with a computer algorithm reduces haemoglobin variability. Nephrol. Dial. Transplant. 25:2710–2714, 2010.
Kalanta-Zadeh, K. History of erythropoiesis-stimulating agents, the development of biosimilars, and the future of anemia treatment in nephrology. Am. J. Nephrol. 45(3):235–247, 2017.
Kanbay, M., M. A. Perazella, B. Kasapoglu, M. Koroglu, and A. Covic. Erythropoiesis stimulatory agent- resistant anemia in dialysis patients: review of causes and management. Blood Purif. 29(1):1–12, 2010.
Krzyzanski, W., S. Woo, and W. J. Jusko. Pharmacodynamic models for agents that alter production of natural cells with various distributions of lifespans. J. Pharmacokinet. Pharmacody. 33(2):125–166, 2006.
Lines, S. W., E. J. Lindley, J. E. Tattersall, and M. J. Wright. A predictive algorithm for the management of anaemia in haemodialysis patients based on ESA pharmacodynamics: better results for less work. Nephrol. Dial. Transplant. 27(6):2425–2429, 2012.
National Kidney Foundation, Inc., KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease, http://www.kidney.org/professionals/KDOQI/guidelines_anemia/index.htm, 2006.
Pottgiesser, T., W. Specker, M. Umhau, H. H. Dickhuth, K. Roecker, and Y. O. Schumacher. Recovery of hemoglobin mass after blood donation. Transfusion. 48(7):1390–1397, 2008.
Schneider, A., A. Gernot, P. Biggar, J. Braun, F. Dellanna, R. Fiedler, J. Galle, M. Girndt, K. Gondolf, K. Hahn, M. Koch, H. J. Müller, L. C. Rump, A. Vosskühler, R. Winkler, and C. Wanner. Hemoglobin cycling in hemodialysis patients. Nephrol. Rev. 2(e1):1–5, 2010.
Shrestha, R. P., J. Horowitz, C. V. Hollot, M. J. Germain, J. A. Widness, D. M. Mock, P. Veng-Pedersen, and Y. Chait. Models for the red blood cell lifespan. J Pharmacokinet. Pharmacodyn. 43(3):259–274, 2016.
Singh, A. K., E. Milford, S. Fishbane, and S. R. Keithi-Reddy. Managing anemia in dialysis patients: hemoglobin cycling and overshoot. Kidney Int. 74(5):679–683, 2008.
Thakuria, M., N. J. Ofsthun, C. Mullon, and J. A. Diaz-Buxo. Anemia management in patients receiving chronic hemodialysis. Semin. Dial. 24(5):597–602, 2011.
Uehlinger, D. E., F. A. Gotch, and L. B. Sheiner. A pharmacodynamic model of erythropoietin therapy for uremic anemia. Clin. Pharmacol. Ther. 51(1):76–89, 1992.
Acknowledgments
Dr. Chait would like to express his gratitude to Dr. Adam Gaweda for discussions of his experiences in anemia management. We would like to thank Dr. Alex Biro (study director in Israel) and anemia and clinical managers at the various sites. This work was supported in part by a grant from National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (K25 DK096006 to YC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Erythropoiesis Modelling in ESRD
Appendix: Erythropoiesis Modelling in ESRD
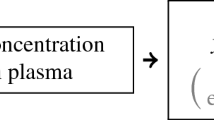
The dynamics of Hb concentration following the administration of intravenous ESA has been described using a combination of pharmacokinetic (PK) and pharmacodynamics (PD) models.8,23,31 We present the PK (top two relations below) and the production portion of the PD together: for t ≥ 0,
where \( V \), \( K_{\text{m}} \), C and \( S \) are constants, \( \alpha \) is the linear clearance constant, \( k_{\text{in}} \) is the RBC production-rate, \( E_{P} \) denotes EPO in plasma, \( E_{\text{en}} \) is endogenous EPO, and \( E \) is exogenous ESA. The term \( {\text{dose}}(t) \) denotes the ESA dose at time t, which we model as a train of impulses of ESA doses.
The remaining part of the PD model is for the RBC population R(t) at time t ≥ 0, given by8
se
where \( \ell (\tau ) \) is the probability density function (pdf) of the lifespan \( \tau \) of an RBC entering the pool at any time t, \( D \) is the total time required for EPO-stimulated progenitor cells to progress through their various stages and finally become reticulocytes ready to mature into RBCs, \( R_{0} \) denotes the initial RBC population at t = 0, at which time EPO therapy begins, and \( K_{\text{H}} \) is the average amount of [Hb] per RBC (mean corpuscular hemoglobin, or MCH, in a complete blood count). We initially assumed that \( \ell (\tau ) \) is a time-invariant 2nd-order gamma pdf
where \( \mu \) is the mean RBC lifespan. This assumption was later confirmed in a clinical study.28
We used retrospective data from 44 HD patients collected over a 16-month period20 to establish suitability of this model. The data consisted of administered rHuEPO doses and Hb measurements. Each patient’s model was identified using a training period of ≥ 90 days, which is consistent with the reported range of RBC lifespans of 34–120 days in ESRD patients.31 Each training period was followed by a validation period of similar length. We illustrate estimation results of the parameters in the above nonlinear model corresponding to patient #27 in Fig. 9. This figure shows a training period (days 82–277, shaded in the figure) followed by a validation period (days 278–473) during which the estimated model accurately predicts the actual response, although the match degrades slightly over time. Note that this training/validation sequence followed an initial training period (days 0–82) that produced a model which failed to predict the response beyond that period, and so was re-initiated at day 82. There are several possible explanations for this: (a) the training period was too short relative to the dynamics of the RBC pool (approximated by the RBC lifespan, 92.2 days), and (b) the rapid decrease in Hb evident during days 0–82, occurring during administration of a constant rHuEPO dose, may indicate an event not included in the model, such as gastrointestinal bleeding.
Rights and permissions
About this article
Cite this article
Chait, Y., Germain, M.J., Hollot, C.V. et al. The Role of Feedback Control Design in Developing Anemia Management Protocols. Ann Biomed Eng 49, 171–179 (2021). https://doi.org/10.1007/s10439-020-02520-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02520-1