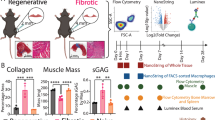
Abstract
Numerous studies have pharmacologically modulated the muscle milieu in the hopes of promoting muscle regeneration; however, the timing and duration of these interventions are difficult to determine. This study utilized a combination of in silico and in vivo experiments to investigate how inflammation manipulation improves muscle recovery following injury. First, we measured macrophage populations following laceration injury in the rat tibialis anterior (TA). Then we calibrated an agent-based model (ABM) of muscle injury to mimic the observed inflammation profiles. The calibrated ABM was used to simulate macrophage and satellite stem cell (SC) dynamics, and suggested that delivering macrophage colony stimulating factor (M-CSF) prior to injury would promote SC-mediated injury recovery. Next, we performed an experiment wherein 1 day prior to injury, we injected M-CSF into the rat TA muscle. M-CSF increased the number of macrophages during the first 4 days post-injury. Furthermore, treated muscles experienced a swifter increase in the appearance of PAX7+ SCs and regenerating muscle fibers. Our study suggests that computational models of muscle injury provide novel insights into cellular dynamics during regeneration, and further, that pharmacologically altering inflammation dynamics prior to injury can accelerate the muscle regeneration process.






Similar content being viewed by others
References
Allen, R. E., and L. K. Boxhorn. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J. Cell. Physiol. 133:567–572, 1987.
Allen, R. E., and L. K. Boxhorn. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J. Cell. Physiol. 138:311–315, 1989.
Arnold, L., A. Henry, F. Poron, Y. Baba-Amer, N. van Rooijen, A. Plonquet, R. K. Gherardi, and B. Chazaud. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204:1057–1069, 2007.
Bailey, A. M., B. C. Thorne, and S. M. Peirce. Multi-cell agent-based simulation of the microvasculature to study the dynamics of circulating inflammatory cell trafficking. Ann. Biomed. Eng. 35:916–936, 2007.
Bencze, M., E. Negroni, D. Vallese, H. Yacoub-Youssef, S. Chaouch, A. Wolff, A. Aamiri, J. P. Di Santo, B. Chazaud, G. Butler-Browne, W. Savino, V. Mouly, and I. Riederer. Proinflammatory macrophages enhance the regenerative capacity of human myoblasts by modifying their kinetics of proliferation and differentiation. Mol. Ther. 20:2168–2179, 2012.
Bentzinger, C. F., Y. X. Wang, N. A. Dumont, and M. A. Rudnicki. Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 14:1062–1072, 2013.
Borselli, C., H. Storrie, F. Benesch-Lee, D. Shvartsman, C. Cezar, J. W. Lichtman, H. H. Vandenburgh, and D. J. Mooney. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl. Acad. Sci. U.S.A. 107:3287–3292, 2010.
Brigitte, M., C. Schilte, A. Plonquet, Y. Baba-Amer, A. Henri, C. Charlier, S. Tajbakhsh, M. Albert, R. K. Gherardi, and F. Chrétien. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum. 62:268–279, 2010.
Chazaud, B., C. Sonnet, P. Lafuste, G. Bassez, A.-C. C. Rimaniol, F. Poron, F.-J. Authier, P. A. Dreyfus, and R. K. Gherardi. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell Biol. 163:1133–1143, 2003.
Cheung, E. V., and J. G. Tidball. Administration of the non-steroidal anti-inflammatory drug ibuprofen increases macrophage concentrations but reduces necrosis during modified muscle use. Inflamm. Res. 52:170–176, 2003.
Christ, G. J., J. M. Saul, M. E. Furth, and K.-E. Andersson. The pharmacology of regenerative medicine. Pharmacol. Rev. 65:1091–1133, 2013.
Christov, C., F. Chretien, R. Abou-Khalil, G. Bassez, G. Vallet, F.-J. Authier, Y. Bassaglia, V. Shinin, S. Tajbakhsh, B. Chazaud, and R. K. Gherardi. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol. Biol. Cell 17:1397–1409, 2007.
Corliss, B. A., M. S. Azimi, J. Munson, S. M. Peirce, and W. L. Murfee. Macrophages: an inflammatory link between angiogenesis and lymphangiogenesis. Microcirculation 23:95–121, 2016.
Corona, B. T., C. L. Ward, H. B. Baker, T. J. Walters, and G. J. Christ. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng. Part A 20:705–715, 2013.
Côté, C. H., P. Bouchard, N. van Rooijen, D. Marsolais, and E. Duchesne. Monocyte depletion increases local proliferation of macrophage subsets after skeletal muscle injury. BMC Musculoskelet. Disord. 14:359, 2013.
Deng, B., M. Wehling-Henricks, S. A. Villalta, Y. Wang, and J. G. Tidball. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 189:3669–3680, 2012.
Dumont, N. A., and J. Frenette. Macrophage colony-stimulating factor-induced macrophage differentiation promotes regrowth in atrophied skeletal muscles and C2C12 myotubes. Am. J. Pathol. 182:505–515, 2013.
Fadok, V. A., D. L. Bratton, A. Konowal, P. W. Freed, J. Y. Westcott, and P. M. Henson. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101:890–898, 1998.
Fujita, R., F. Kawano, T. Ohira, N. Nakai, T. Shibaguchi, N. Nishimoto, Y. Ohira, and R. Fujita. Anti-interleukin-6 receptor antibody (MR16-1) promotes muscle regeneration via modulation of gene expressions in infiltrated macrophages. Biochim. Biophys. Acta 1840(10):3170–3180, 2014.
Fukushima, K., N. Badlani, A. Usas, F. Riano, F. Fu, and J. Huard. The use of an antifibrosis agent to improve muscle recovery after laceration. Am. J. Sports Med. 29:394–402, 2001.
Germani, A., A. Di Carlo, A. Mangoni, S. Straino, C. Giacinti, P. Turrini, P. Biglioli, and M. C. Capogrossi. Vascular endothelial growth factor modulates skeletal myoblast function. Am. J. Pathol. 163:1417–1428, 2003.
Gopalakrishnan, V., M. Kim, and G. An. Using an agent-based model to examine the role of dynamic bacterial virulence potential in the pathogenesis of surgical site infection. Adv. Wound Care 2:510–526, 2013.
Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23–35, 2003.
Gordy, C., H. Pua, G. D. Sempowski, and Y. W. He. Regulation of steady-state neutrophil homeostasis by macrophages. Blood 117:618–629, 2011.
Hamilton, J. A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8:533–544, 2008.
Hara, M., S. Yuasa, K. Shimoji, T. Onizuka, N. Hayashiji, Y. Ohno, T. Arai, F. Hattori, R. Kaneda, K. Kimura, S. Makino, M. Sano, and K. Fukuda. G-CSF influences mouse skeletal muscle development and regeneration by stimulating myoblast proliferation. J. Exp. Med. 208:715–727, 2011.
Hurme, T., and H. Kalimo. Activation of myogenic precursor cells after muscle injury. Med. Sci. Sports Exerc. 24:197–205, 1992.
Järvinen, T. A. H., T. L. N. Järvinen, M. Kääriäinen, H. Kalimo, and M. Järvinen. Muscle injuries: biology and treatment. Am. J. Sports Med. 33:745–764, 2005.
Kaplanski, G., V. Marin, F. Montero-Julian, A. Mantovani, and C. Farnarier. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 24:25–29, 2003.
Lu, H., D. Huang, N. Saederup, I. F. Charo, R. M. Ransohoff, and L. Zhou. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 25:358–369, 2011.
Maas, H., and P. A. Huijing. Effects of tendon and muscle belly dissection on muscular force transmission following tendon transfer in the rat. J. Biomech. 45:289–296, 2012.
Martin, K. S., K. M. Virgilio, S. M. Peirce, and S. S. Blemker. Computational modeling of muscle regeneration and adaptation to advance muscle tissue regeneration strategies. Cells Tissues Organs, in press, 2016. http://www.karger.com/Book/Home/272023.
Martin, K. S., S. S. Blemker, and S. M. Peirce. Agent-based computational model investigates muscle-specific responses to disuse-induced atrophy. J. Appl. Physiol. 118:1299–1309, 2015.
McLennan, I. S. Degenerating and regenerating skeletal muscles contain several subpopulations of macrophages with distinct spatial and temporal distributions. J. Anat. 188:17–28, 1996.
Meneghini, R. M., M. W. Pagnano, R. T. Trousdale, and W. J. Hozack. Muscle damage during MIS total hip arthroplasty: Smith-Petersen versus posterior approach. Clin. Orthop. Relat. Res. 453:293–298, 2006.
Menetrey, J., C. Kasemkijwattana, C. S. Day, P. Bosch, M. Vogt, F. H. Fu, M. S. Moreland, and J. Huard. Growth factors improve muscle healing in vivo. J. Bone Joint Surg. Br. 82:131–137, 2000.
Miller, K. J., D. Thaloor, S. Matteson, and G. K. Pavlath. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am. J. Physiol. Cell Physiol. 278:C174–C181, 2000.
Mosser, D. M., and J. P. Edwards. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8:958–969, 2008.
Motohashi, N., A. Uezumi, E. Yada, S. Fukada, K. Fukushima, K. Imaizumi, Y. Miyagoe-Suzuki, and S. Takeda. Muscle CD31(−) CD45(−) side population cells promote muscle regeneration by stimulating proliferation and migration of myoblasts. Am. J. Pathol. 173:781–791, 2008.
Mueller, M., C. Leonhard, K. Wacker, E. B. Ringelstein, M. Okabe, W. F. Hickey, and R. Kiefer. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab. Investig. 83:175–185, 2003.
Muñoz-Cánoves, P., C. Scheele, B. K. Pedersen, and A. L. Serrano. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 280:4131–4148, 2013.
Murphy, M. M., J. A. Lawson, S. J. Mathew, D. A. Hutcheson, and G. Kardon. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138:3625–3637, 2011.
Nacu, N., I. G. Luzina, K. Highsmith, V. Lockatell, K. Pochetuhen, Z. A. Cooper, M. P. Gillmeister, N. W. Todd, and S. P. Atamas. Macrophages produce TGF-beta-induced (beta-ig-h3) following ingestion of apoptotic cells and regulate MMP14 levels and collagen turnover in fibroblasts. J. Immunol. 180:5036–5044, 2008.
Novak, M. L., and T. J. Koh. Macrophage phenotypes during tissue repair. J. Leukoc. Biol. 93:875–881, 2013.
Nozaki, M., Y. Li, J. Zhu, F. Ambrosio, K. Uehara, F. H. Fu, and J. Huard. Improved muscle healing after contusion injury by the inhibitory effect of suramin on myostatin, a negative regulator of muscle growth. Am. J. Sports Med. 36:2354–2362, 2008.
Rossi, R., A. Maiello, M. Bruzzone, D. E. Bonasia, D. Blonna, and F. Castoldi. Muscle damage during minimally invasive surgical total knee arthroplasty traditional versus optimized subvastus approach. Knee 18:254–258, 2011.
Sato, K., Y. Li, W. Foster, K. Fukushima, N. Badlani, N. Adachi, A. Usas, F. H. Fu, and J. Huard. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle and Nerve 28:365–372, 2003.
Schabort, E. J., M. Van Der Merwe, and C. U. Niesler. TGF-b isoforms inhibit IGF-1-induced migration and regulate terminal differentiation in a cell-specific manner. J. Muscle Res. Cell Motil. 31:359–367, 2011.
Serrano, A. L., B. Baeza-Raja, E. Perdiguero, M. Jardí, and P. Muñoz-Cánoves. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 7:33–44, 2008.
Shen, W., V. Prisk, Y. Li, W. Foster, and J. Huard. Inhibited skeletal muscle healing in cyclooxygenase-2 gene-deficient mice: the role of PGE 2 and PGF 2alpha. J. Appl. Physiol. 101:1215–1221, 2006.
Siegel, A. L., K. Atchison, K. E. Fisher, G. E. Davis, and D. D. W. Cornelison. 3D timelapse analysis of muscle satellite cell motility. Stem Cells 27:2527–2538, 2009.
Smeulders, M. J. C., and M. Kreulen. Myofascial force transmission and tendon transfer for patients suffering from spastic paresis: a review and some new observations. J. Electromyogr. Kinesiol. 17:644–656, 2007.
Song, E., N. Ouyang, M. Hörbelt, B. Antus, M. Wang, and M. S. Exton. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell. Immunol. 204:19–28, 2000.
Spitzer, M. H., P. F. Gherardini, G. K. Fragiadakis, N. Bhattacharya, R. T. Yuan, A. N. Hotson, R. Finck, Y. Carmi, E. R. Zunder, W. J. Fantl, S. C. Bendall, E. G. Engleman, and G. P. Nolan. An interactive reference framework for modeling a dynamic immune system. Science 349:1259425, 2015.
Strle, K., S. R. Broussard, R. H. McCusker, W. H. Shen, R. W. Johnson, G. G. Freund, R. Dantzer, and K. W. Kelley. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology 145:4592–4602, 2004.
Strle, K., R. H. McCusker, L. Tran, A. King, R. W. Johnson, G. G. Freund, R. Dantzer, and K. W. Kelley. Novel activity of an anti-inflammatory cytokine: IL-10 prevents TNFα-induced resistance to IGF-I in myoblasts. J. Neuroimmunol. 188:48–55, 2007.
Takeuchi, K., T. Hatade, S. Wakamiya, N. Fujita, T. Arakawa, and A. Miki. Heat stress promotes skeletal muscle regeneration after crush injury in rats. Acta Histochem. 116:327–334, 2014.
Thorne, B. C., A. M. Bailey, D. W. DeSimone, and S. M. Peirce. Agent-based modeling of multicell morphogenic processes during development. Birth Defects Res. C. Embryo Today 81:344–353, 2007.
Tidball, J. G., and S. A. Villalta. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298:R1173–R1187, 2010.
Wang, H., D. W. Melton, L. Porter, Z. U. Sarwar, L. M. McManus, and P. K. Shireman. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am. J. Pathol. 184:1167–1184, 2014.
Wu, L. Y. L. Yu, R. D. Galiano, S. I. Roth, and T. a Mustoe. Macrophage colony-stimulating factor accelerates wound healing and upregulates TGF-beta1 mRNA levels through tissue macrophages. J. Surg. Res. 72:162–169, 1997.
Yablonka-Reuveni, Z., T. M. Balestreri, and D. F. Bowen-Pope. Regulation of proliferation and differentiation of myoblasts derived from adult mouse skeletal muscle by specific isoforms of PDGF. J. Cell Biol. 111:1623–1629, 1990.
Yin, H., F. Price, and M. A. Rudnicki. satellite cells and the muscle stem cell niche. Physiol. Rev. 93:23–67, 2013.
Zeng, L., Y. Akasaki, K. Sato, N. Ouchi, Y. Izumiya, and K. Walsh. Insulin-like 6 is induced by muscle injury and functions as a regenerative factor. J. Biol. Chem. 285:36060–36069, 2010.
Acknowledgments
This work was supported in part by NSF Grant No. 1235244.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Aleksander S. Popel oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martin, K.S., Kegelman, C.D., Virgilio, K.M. et al. In Silico and In Vivo Experiments Reveal M-CSF Injections Accelerate Regeneration Following Muscle Laceration. Ann Biomed Eng 45, 747–760 (2017). https://doi.org/10.1007/s10439-016-1707-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1707-2