Abstract
The continuing lack of longitudinal histopathological and biomechanical data for human arteries in health and disease highlights the importance of studying the many genetic, pharmacological, and surgical models that are available in mice. As a result, there has been a significant increase in the number of reports on the biomechanics of murine arteries over the past decade, particularly for the common carotid artery. Whereas most of these studies have focused on wild-type controls or comparing controls vs. a single model of altered hemodynamics or vascular disease, there is a pressing need to compare results across many different models to understand more broadly the effects of genetic mutations, pharmacological treatments, or surgical alterations on the evolving hemodynamics and the microstructure and biomechanical properties of these vessels. This paper represents a first step toward this goal, that is, a biomechanical phenotyping of common carotid arteries from control mice and seven different mouse models that represent alterations in elastic fiber integrity, collagen remodeling, and smooth muscle cell functionality.






Similar content being viewed by others
Notes
It would be useful, of course, to compare responses to the same pharmacological treatment or surgical procedure by the different genotypes as well as different arteries for a given phenotype, but this was beyond the present scope.
References
Baek, S., R. L. Gleason, K. R. Rajagopal, and J. D. Humphrey. Theory of small on large: potential utility in computations of fluid-solid interactions in arteries. Comput. Methods Appl. Mech. Eng. 196:3070–3078, 2007.
Bersi, M., M. J. Collins, E. Wilson, and J. D. Humphrey. Disparate changes in the mechanical properties of murine carotid arteries and aorta in response to chronic infusion of angiotensin-II. Int. J. Adv. Eng. Sci. Appl. Math. 4:228–240, 2012.
Cassis, L. A., M. Gupte, S. Thayer, X. Zhang, R. Charnigo, D. A. Howatt, D. L. Rateri, and A. Daugherty. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am. J. Physiol. 296:H1660–H1665, 2009.
Cheng, J. K., I. Stoilov, R. P. Mecham, and J. E. Wagenseil. A fiber-based constitutive model predicts changes in amount and organization of matrix proteins with development and disease in the mouse aorta. Biomech. Model. Mechanobiol. 12:497–510, 2013.
Chung, A. W. Y., K. A. Yeung, G. G. S. Sandor, D. P. Judge, H. C. Dietz, and C. van Breemen. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in thoracic aortic aneurysm in Marfan syndrome. Circ. Res. 101:512–522, 2007.
Cox, R. H. Regional variation of series elasticity in canine arterial smooth muscle. Am. J. Physiol. 234:H542–H551, 1978.
Dajnowiec, D., and B. L. Langille. Arterial adaptations to chronic changes in haemodynamic function: coupling vasomotor tone to structural remodeling. Clin. Sci. 113:15–23, 2007.
Daugherty, A., D. L. Rateri, I. F. Charos, A. P. Owens, D. A. Howatt, and L. A. Cassis. Angiotensin II infusion promotes ascending aortic aneurysms: attenuation by CCR2 deficiency in ApoE −/− mice. Clin. Sci. 118:681–689, 2011.
Dobrin, P. B. Biaxial anisotropy of dog carotid artery: estimation of circumferential elastic modulus. J. Biomech. 19:351–358, 1986.
Dye, W. W., R. L. Gleason, E. Wilson, and J. D. Humphrey. Biaxial biomechanical behavior of carotid arteries in two knockout models of muscular dystrophy. J. Appl. Physiol. 103:664–672, 2007.
Eberth, J. F., A. I. Taucer, E. Wilson, and J. D. Humphrey. Mechanics of carotid arteries from a mouse model of Marfan Syndrome. Ann. Biomed. Eng. 37:1093–1104, 2009.
Eberth, J. F., N. Popovic, V. Gresham, E. Wilson, and J. D. Humphrey. Time course of carotid artery growth and remodeling in response to altered pulsatility. Am. J. Physiol. 299:H1875–H1883, 2010.
Eberth, J. F., L. Cardamone, and J. D. Humphrey. Altered mechanical properties of carotid arteries in hypertension. J. Biomech. 44:2532–2537, 2011.
Ferruzzi, J., M. J. Collins, A. T. Yeh, and J. D. Humphrey. Mechanical assessment of elastin integrity in fibrillin-1 deficient carotid arteries: implications for Marfan syndrome. Cardiovasc. Res. 92:287–295, 2011.
Ferruzzi, J., M. R. Bersi, and J. D. Humphrey. Biomechanical phenotyping of central arteries in health and disease: advantages of and methods for murine models. Ann. Biomed. Eng. 41:1311–1330, 2013.
Fleenor, B. S., K. D. Marshall, J. R. Durrant, L. A. Lesniewski, and D. R. Seals. Arterial stiffening with ageing is associated with transforming growth factor beta 1 related changes in adventitial collagen: reversal by aerobic exercise. J. Physiol. 588:3971–3982, 2010.
Geddes, L. A., M. H. Voelz, C. F. Babbs, J. D. Gourland, and W. A. Tacker. Pulse transit time as an indicator of arterial blood pressure. Psychophysiology 18:71–74, 1981.
Genovese, K., M. J. Collins, Y. U. Lee, and J. D. Humphrey. Regional finite strains in an angiotensin-II infusion model of dissecting abdominal aortic aneurysms. J. Cardiovasc. Eng. Tech. 3:194–202, 2012.
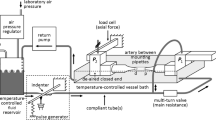
Gleason, R. L., S. P. Gray, E. Wilson, and J. D. Humphrey. A multiaxial computer-controlled organ culture and biomechanical device for mouse carotid arteries. ASME J. Biomech. Eng. 126:787–795, 2004.
Gleason, R. L., W. W. Dye, E. Wilson, and J. D. Humphrey. Quantification of the mechanical behavior of carotid arteries from wild-type, dystrophin-deficient, and sarcoglycan-delta knockout mice. J. Biomech. 41:3213–3218, 2008.
Greve, J. M., A. S. Les, B. T. Tang, M. T. Draney Blomme, N. M. Wilson, R. L. Dalman, N. J. Pelc, and C. A. Taylor. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am. J. Physiol. 291:H1700–H1708, 2006.
Hanada, K., M. Vermeij, G. A. Garinis, M. C. de Waard, M. G. S. Kunen, L. Myers, A. Maas, D. J. Duncker, C. Meijers, H. C. Deitz, R. Kanaar, and J. Essers. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circ. Res. 100:738–746, 2007.
Harmon, K. J., L. L. Couper, and V. Lindner. Strain-dependent vascular remodeling phenotypes in inbred mice. Am. J. Path. 156:1741–1748, 2000.
Hartner, A., L. Schaefer, M. Porst, N. Cordasic, A. Gabriel, B. Klanke, D. P. Reinhardt, and K. F. Hilgers. Role of fibrillin-1 in hypertensive and diabetic glomerular disease. Am. J. Physiol. Ren. Physiol. 290:F1329–F1336, 2006.
Haskett, D., J. J. Doyle, C. Gard, H. Chen, C. Ball, M. A. Estabrook, A. C. Encinas, H. C. Dietz, U. Utzinger, J. P. Vande Geest, and M. Azhar. Altered tissue behavior of a non-aneurysmal descending thoracic aorta in the mouse model of Marfan syndrome. Cell Tissue Res. 347:267–277, 2012.
Hayenga, H. N., A. Trache, J. Trzeciakowski, and J. D. Humphrey. Regional atherosclerotic plaque properties in ApoE −/− mice measured by atomic force, immunofluorescence, and light microscopy. J. Vasc. Res. 48:495–504, 2011.
Holzapfel, G. A., T. C. Gasser, and R. W. Ogden. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. 61:1–48, 2000.
Huang, J., E. C. Davis, S. L. Chapman, M. Budatha, L. Y. Marmorstein, R. A. Word, and H. Yanagisawa. Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ. Res. 106:583–592, 2010.
Humphrey, J. D. Cardiovascular solid mechanics. Cells, tissues, and organs. New York: Springer, 2002.
Humphrey, J. D. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem. Biophys. 50:53–78, 2008.
Humphrey, J. D. Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension 52:195–200, 2008.
Humphrey, J. D., J. F. Eberth, W. W. Dye, and R. L. Gleason. Fundamental role of axial stress in compensatory adaptations by arteries. J. Biomech. 42:1–8, 2009.
Humphrey, J. D. Possible roles of glycosaminoglycans in aortic dissections, with implications to dysfunctional TGF-beta. J. Vasc. Res. 50:1–10, 2013.
Janssen, B. J. A., T. De Celle, J. J. M. Debets, A. E. Brouns, M. F. Callahan, and T. L. Smith. Effects of anesthetics on systemic hemodynamics in mice. Am. J. Physiol. Heart Circ. Physiol. 287:H1618–H1624, 2004.
Koullias, G., R. Modak, M. Tranquilli, D. P. Korkolis, P. Barash, and J. A. Elefteriades. Mechanical deterioration underlies malignant behavior of aneurysmal human ascending aorta. J. Thorac. Cardiovasc. Surg. 130:677–683, 2005.
Majesky, M. W. Developmental basis of vascular smooth muscle diversity. Arterioscl. Thromb. Vasc. Biol. 27:1248–1258, 2007.
Marque, V., P. Kieffer, B. Gayraud, I. Lartaud-Idjouadinene, F. Ramirez, and J. Atkinson. Aortic wall mechanics and composition in a transgenic mouse model of Marfan syndrome. Arterioscl. Thromb. Vasc. Biol. 21:1184–1189, 2001.
Masson, I., P. Boutouyrie, S. Laurent, J. D. Humphrey, and M. Zidi. Characterization of arterial wall mechanical properties and stresses from human clinical data. J. Biomech. 41:2618–2627, 2008.
Matlung, H. L., A. E. Neele, H. C. Groen, K. van Gaalen, B. G. Tuna, A. van Weert, J. de Vos, J. J. Wentzel, M. Hoogenboezem, J. D. van Buul, E. Vanbavel, and E. N. Bakker. Transglutaminase activity regulates atherosclerotic plaque composition at locations exposed to oscillatory shear stress. Atherosclerosis 224:355–362, 2012.
Milewicz, D. M., D. C. Guo, V. Tran-Fadulu, A. L. Lafont, C. L. Papke, S. Inamoto, C. S. Kwartler, and H. Pannu. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile function. Annu. Rev. Genomics Hum. Genet. 9:283–302, 2008.
Pereira, L., S. Y. Lee, B. Gayraud, K. Andrikopoulos, S. D. Shapiro, T. Bunton, N. J. Biery, H. C. Dietz, L. Y. Sakai, and F. Ramirez. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc. Natl Acad. Sci. (USA) 96:3819–3823, 1999.
Roccabianca, S., C. A. Figueroa, G. Tellides, and J. D. Humphrey. Quantification of regional differences in aortic stiffness in the aging human aorta. J. Biomech. Behav. Biomed. Matl. 29:618–634, 2014.
Ruiz-Ortega, M., J. Rodriguez-Vita, E. Sanchez-Lopez, G. Carvajal, and J. Egido. TGF-b signaling in vascular fibrosis. Cardiovasc. Res. 74:196–206, 2007.
Sather, B. A., D. Hageman, and J. E. Wagenseil. Murray’s law in elastin haploinsufficient (Eln±) and wild-type (WT) mice. J. Biomech. Eng. 134:124504, 2012.
Schildmeyer, L. A., R. Braun, G. Taffett, M. Debiasi, A. E. Burns, A. Bradley, and R. J. Schwartz. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle α-actin null mouse. FASEB J. 14:2213–2220, 2000.
Stevic, I., H. H. W. Chan, and A. K. C. Chan. Carotid artery dissections: thrombosis of the false lumen. Thromb. Res. 128:317–324, 2011.
Townsend, D., S. Yasuda, E. McNally, and J. M. Metzger. Distinct pathophysiological mechanisms of cardiomyopathy in hearts lacking dystrophin or the sarcoglycan complex. FASEB J. 25:3106–3114, 2011.
Wagenseil, J. E., and R. P. Mecham. Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 89:957–989, 2009.
Wan, W., H. Yanagisawa, and R. L. Gleason. Biomechanical and microstructural properties of common carotid arteries from fibulin-5 null mice. Ann. Biomed. Eng. 38:3605–3617, 2010.
Wan, W., J. B. Dixon, and R. L. Gleason. Constitutive modeling of mouse carotid arteries using experimentally measured microstructural parameters. Biophys. J. 102:2916–2925, 2012.
Wang, X., S. A. LeMaire, L. Chen, et al. Decreased expression of fibulin-5 correlates with reduced elastin in thoracic aortic dissection. Surgery 138:352–359, 2005.
Whitesall, S. E., J. B. Hoff, A. P. Vollmer, and L. G. D’Alecy. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am. J. Physiol. Heart Circ. Physiol. 286:H2408–H2415, 2004.
Wolinsky, H. Effects of hypertension and its reversal on the thoracic aorta of male and female rats. Circ. Res. 28:622–637, 1971.
Yanagisawa, H., E. C. Davis, B. C. Starcher, T. Ouchi, M. Yanagisawa, J. A. Richardson, and E. N. Olson. Fibulin-5 is an elastin-binding protein essential for elastic fiber development in vivo. Nature 415:168–171, 2002.
Yanagisawa, H., and E. C. Davis. Unraveling the mechanism of elastic fiber assembly: the roles of short fibulins. Int. J. Biochem. Cell Biol. 42:1084–1093, 2010.
Zeinali-Davarani, S., J. Choi, and S. Baek. On parameter estimation for biaxial mechanical behavior of arteries. J. Biomech. 42:524–530, 2009.
Acknowledgments
This work was supported, in part, by grants from the National Marfan Foundation and the NIH (R01 HL105297 and R21 HL107768). JDH also acknowledges colleagues (Drs. Vince Gresham, Emily Wilson, and Alvin Yeh at Texas A&M University) and former students (Wendy Dye, M.S., Heather N. Hayenga, Ph.D., Anne I. Taucer, M.S., and Melissa J. Collins, Ph.D.) who contributed so much over the years to this overall work on arterial mechanics in mice. Finally, we are grateful to Dr. Kevin Campbell (HHMI and University of Utah), Dr. Francesco Ramirez (Mt. Sinai School of Medicine), Dr. Warren Zimmer (Texas A&M Health Science Center), and Dr. Hiromi Yanagisawa (University of Texas Southwestern Medical Center) for graciously providing the initial breeding pairs for the Sgcd −/−, Fbn1 mgR/mgR, Acta2 −/−, and Fbln5 −/− mice, respectively.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor K. A. Athanasiou oversaw the review of this article.
M. R. Bersi and J. Ferruzzi contributed equally to this work.
Appendix
Appendix
Reported values of systolic blood pressure summarized in Table 1 were collected using different methods, with data for control, Ang-II ApoE −/−, and Acta2 −/− mice measured using a noninvasive tail cuff method in the conscious mouse,3,24,45 data for the Fbn1 mgR/mgR and Fbln5 −/− mice measured via an indwelling polyethylene catheter in the conscious mouse,37,54 and data for the mdx and Sgcd −/− mice measured under isoflurane anesthesia using a left ventricular catheter.47 To render more consistent the comparison of intramural stress, stiffness, and stored energy across mouse models, these pressures were adjusted to central arterial pressure in the conscious state using published correlations.34,52 Briefly, Whitesall and colleagues simultaneously measured pressure using direct (radiotelemetry) and indirect (noninvasive tail-cuff) methods and reported a linear relationship, P TC = 0.961 × P C + 4.203 mmHg, where P TC and P C are systolic pressures measured by tail-cuff and telemetry (or central pressure), respectively. Rearranging this equation, tail-cuff measured pressures (i.e., control, Ang-II ApoE −/−, and Acta2 −/− mice) were adjusted to the conscious central pressure state. In contrast, Janssen and colleagues used an indwelling central artery catheter to evaluate effects of multiple anesthetics on the hemodynamics in mice. They found that isoflurane reduces the measured pressure by 24.3% relative to conscious ambulatory pressures. Hence, we adjusted pressures measured under isoflurane anesthesia (i.e., mdx and Sgcd −/−) via P A = 0.757 × P C, where P A represents the anesthetized pressure. Table 1 thus lists both the value reported in the respective paper and the values adjusted herein.
Rights and permissions
About this article
Cite this article
Bersi, M.R., Ferruzzi, J., Eberth, J.F. et al. Consistent Biomechanical Phenotyping of Common Carotid Arteries from Seven Genetic, Pharmacological, and Surgical Mouse Models. Ann Biomed Eng 42, 1207–1223 (2014). https://doi.org/10.1007/s10439-014-0988-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-0988-6