Abstract
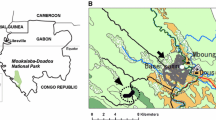
The Taï region in Western Côte d`Ivoire is characterized by extensive overlap of human and animal habitats. This could influence patterns of adenovirus transmission between humans and domestic animals. Fecal samples from humans and various domestic animals were tested for the presence of adenoviruses by PCR. Phylogenetic and species delineation analyses were performed to further characterize the adenoviruses circulating in the region and to identify potential cross-species transmission events. Among domestic animals, adenovirus shedding was frequent (21.6% of domestic mammals and 41.5% of chickens) and the detected strains were highly diverse, several of them representing novel types. Although no evidence for zoonotic transmission of animal adenovirus was obtained, the present study provides concordant evidence in favor of common cross-species transmission of adenoviruses between different animal species and first indications for adenovirus transmission from humans to animals. These findings underline the thus far underestimated importance of reverse zoonotic transmission of viruses and of the role of domestic animals as pathogen reservoirs, “bridge species,” or intermediate hosts.



Similar content being viewed by others
References
Barbezange C, Benko M, Dán Á, and Harrach B (2000). DNA sequencing and phylogenetic analysis of the protease gene of ovine adenovirus 3 suggest that adenoviruses of sheep belong to two different genera. Virus Research 66:79-85.
Belák, and Pálfi (1974). An adenovirus isolated from sheep and its relationship to type 2 bovine adenovirus. Archiv für die gesamte Virusforschung 46:366-369.
Benkö M, and Harrach B (2003). Molecular evolution of adenoviruses. Current Topics in Microbiology and Immunology 272:3-35.
Bouckaert R, Heled J, Kuhnert D, Vaughan T, Wu CH, Xie D, et al. (2014). BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS computational biology 10:e1003537.
Brister JR, Chodosh J, Curiel DT, Heim A, Jones MS, Kajon A, et al. (2009) Human adenovirus genotype classification. Available: http://hadvwg.gmu.edu/. Accessed on June 15, 2014.
Castresana J (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular biology and evolution 17:540-552.
Chan EH, Brewer TF, Madoff LC, Pollack MP, Sonricker AL, Keller M, et al. (2010). Global capacity for emerging infectious disease detection. Proceedings of the National Academy of Sciences of the United States of America 107:21701-21706.
Chen EC, Yagi S, Kelly KR, Mendoza SP, Tarara RP, Canfield DR, et al. (2011). Cross-species transmission of a novel adenovirus associated with a fulminant pneumonia outbreak in a new world monkey colony. PLoS pathogens 7:e1002155.
Chiu CY, Yagi S, Lu X, Yu G, Chen EC, Liu M, et al. (2013). A novel adenovirus species associated with an acute respiratory outbreak in a baboon colony and evidence of coincident human infection. MBio 4:e00084.
Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, et al. (2000). Nipah virus: a recently emergent deadly paramyxovirus. Science 288:1432-1435.
Cleaveland S, Laurenson MK, and Taylor LH (2001). Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philosophical Transactions of the Royal Society B: Biological Sciences 356:991-999.
Cleaveland S, Meslin FX, and Breiman R (2006). Dogs can play useful role as sentinel hosts for disease. Nature 440:605.
Darriba D, Taboada GL, Doallo R, and Posada D (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature methods 9:772.
Daszak P, Cunningham AA, and Hyatt AD (2000). Emerging infectious diseases of wildlife – threats to biodiversity and human health. Science 287:443-449.
Davison AJ, Benkö M, and Harrach B (2003). Genetic content and evolution of adenoviruses. J Gen Virol 84:2895-2908.
Drummond AJ, Suchard MA, Xie D, and Rambaut A (2012). Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular biology and evolution 29:1969-1973.
Duffy G, and Moriarty EM (2003). Cryptosporidium and its potential as a food-borne pathogen. Animal health research reviews 4:95-107.
Echavarria M (2008). Adenoviruses in immunocompromised hosts. Clinical Microbiology Reviews 21:704-715.
Fujisawa T, and Barraclough TG (2013). Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent approach: a revised method and evaluation on simulated data sets. Systematic biology 62:707-724.
Gouy M, Guindon S, and Gascuel O (2010). SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular biology and evolution 27:221-224.
Guindon S, and Gascuel O (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic biology 52:696-704.
Halliday J, Daborn C, Auty H, Mtema Z, Lembo T, Bronsvoort BM, et al. (2012). Bringing together emerging and endemic zoonoses surveillance: shared challenges and a common solution. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 367:2872-2880.
Harrach B, Benkö M, Both GW, Brown M, Davison AJ, Echavarría M, et al. (2011). Virus Taxonomy. Elsevier, Oxford.
Harrach B, Turnell AS, Leppard KN, and Benkö M (2008). Adenoviruses. in Encyclopedia of Virology B. W. J. Mahy and M. H. V. van Regenmortel, editors. Academic Press, London, Pp 1-24.
Horwitz M., Wold W. (2007) Adenoviruses. in Fields Virology. Knipe D. M. and P. M. Howley, editors. Lippincott William Wilkins, Philadelphia. pp. 2395-2436.
Intisar KS, Ali YH, Khalafalla AI, Taha KM, and Rahman MEA (2010). Adenovirus type 3 infections in camels in Sudan. African Journal of Microbiology Research Vol. 4:1356-1358.
Jones BA, Grace D, Kock R, Alonso S, Rushton J, Said MY, et al. (2013). Zoonosis emergence linked to agricultural intensification and environmental change. Proceedings of the National Academy of Sciences of the United States of America 110:8399-8404.
Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. (2008). Global trends in emerging infectious diseases. Nature 451:990-993.
Kajan GL, Kecskemeti S, Harrach B, and Benko M (2013). Molecular typing of fowl adenoviruses, isolated in Hungary recently, reveals high diversity. Veterinary Microbiology 167:357-363.
Kojaoghlanian T, Flomenberg P, and Horwitz MS (2003). The impact of adenovirus infection on the immunocompromised host. Reviews in Medical Virology 13:155-171.
Lehmkuhl HD, and Hobbs LA (2008). Serologic and hexon phylogenetic analysis of ruminant adenoviruses. Archives of virology 153:891-897.
Lim TH, Lee HJ, Lee DH, Lee YN, Park JK, Youn HN, et al. (2011). Identification and virulence characterization of fowl adenoviruses in Korea. Avian diseases 55:554-560.
Marek A, Gunes A, Schulz E, and Hess M (2010). Classification of fowl adenoviruses by use of phylogenetic analysis and high-resolution melting-curve analysis of the hexon L1 gene region. Journal of virological methods 170:147-154.
Martin DP, Lemey P, Lott M, Moulton V, Posada D, and Lefeuvre P (2010). RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics 26:2462-2463.
Martin DP, Williamson C, and Posada D (2005). RDP2: recombination detection and analysis from sequence alignments. Bioinformatics 21:260-262.
Mase M, Chuujou M, Inoue T, Nakamura K, Yamaguchi S, and Imada T (2009). Genetic characterization of fowl adenoviruses isolated from chickens with hydropericardium syndrome in Japan. The Journal of veterinary medical science 71:1455-1458.
Messenger AM, Barnes AN, and Gray GC (2014). Reverse zoonotic disease transmission (zooanthroponosis): a systematic review of seldom-documented human biological threats to animals. PLoS One 9:e89055.
Meulemans G, Boschmans M, Berg TP, and Decaesstecker M (2001). Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses. Avian Pathology 30:655-660.
Ojkic D, Martin E, Swinton J, Vaillancourt JP, Boulianne M, and Gomis S (2008). Genotyping of Canadian isolates of fowl adenoviruses. Avian Pathology 37:95-100.
Pauly M, Hoppe E, Mugisha L, Petrzelkova K, Akoua-Koffi C, Couacy-Hymann E, et al. (2014). High prevalence and diversity of species D adenoviruses (HAdV-D) in human populations of four Sub-Saharan countries. Virology Journal 11:25.
Pearce-Duvet JM (2006). The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease. Biological reviews of the Cambridge Philosophical Society 81:369-382.
Pons J, Barraclough TG, Gomez-Zurita J, Cardoso A, Duran DP, Hazell S, et al. (2006). Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Systematic biology 55:595-609.
R-Core-Team (2014). A Language and Environment for Statistical Computing. R F S Computing, Vienna.
Rimmelzwaan GF, van Riel D, Baars M, Bestebroer TM, van Amerongen G, Fouchier RA, et al. (2006). Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. The American Journal of Pathology 168:176-183.
Robinson CM, Zhou X, Rajaiya J, Yousuf MA, Singh G, DeSerres JJ, et al. (2013). Predicting the next eye pathogen: analysis of a novel adenovirus. MBio 4:e00595-00612.
Roy S, Sandhu A, Medina A, Clawson DS, and Wilson JM (2012). Adenoviruses in fecal samples from asymptomatic rhesus macaques, United States. Emerging infectious diseases 18:1081-1088.
Sibley SD, Goldberg TL, and Pedersen JA (2011). Detection of known and novel adenoviruses in cattle wastes via broad-spectrum primers. Applied and environmental microbiology 77:5001-5008.
Steer PA, Kirkpatrick NC, O’Rourke D, and Noormohammadi AH (2009). Classification of fowl adenovirus serotypes by use of high-resolution melting-curve analysis of the hexon gene region. Journal of clinical microbiology 47:311-321.
Talavera G, and Castresana J (2007). Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic biology 56:564-577.
Thompson JD, Higgins DG, and Gibson TJ (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic acids research 22:4673-4680.
Vestheim H, and Jarman SN (2008). Blocking primers to enhance PCR amplification of rare sequences in mixed samples - a case study on prey DNA in Antarctic krill stomachs. Frontiers in zoology 5:12.
Walsh MP, Seto J, Jones MS, Chodosh J, Xu W, and Seto D (2010). Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. Journal of clinical microbiology 48:991-993.
Wevers D, Metzger S, Babweteera F, Bieberbach M, Boesch C, Cameron K, et al. (2011). Novel adenoviruses in wild primates: a high level of genetic diversity and evidence of zoonotic transmissions. Journal of virology 85:10774-10784.
Wood JL, Leach M, Waldman L, Macgregor H, Fooks AR, Jones KE, et al. (2012). A framework for the study of zoonotic disease emergence and its drivers: spillover of bat pathogens as a case study. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 367:2881-2892.
Yu G, Yagi S, Carrion R, Jr., Chen EC, Liu M, Brasky KM, et al. (2013). Experimental cross-species infection of common marmosets by titi monkey adenovirus. PLoS One 8:e68558.
Acknowledgments
We thank all the people who volunteered to participate in this study as study participant or by providing the animals for sample collection. Special thanks go also to the sampling team during the field missions (among others Bozua, Ange Hermann Gnoukpoho, Eric Goueu, Joel Semporé and Dan Driscoll). Moreover, we are grateful to Sonja Liebmann, Ulla Thiesen, and Nezlisah Yasmum for their support and assistance. We also thank the national and local health authorities in Côte d’Ivoire, as well as the according ethics commission for granting permission for this work. This work was partly supported by the “Deutsche Forschungsgemeinschaft” (DFG) Grant LE1813/4-1.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pauly, M., Akoua-Koffi, C., Buchwald, N. et al. Adenovirus in Rural Côte D`Ivoire: High Diversity and Cross-Species Detection. EcoHealth 12, 441–452 (2015). https://doi.org/10.1007/s10393-015-1032-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-015-1032-5