Abstract
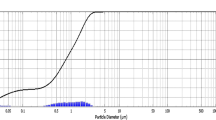
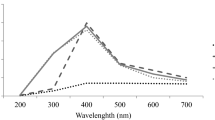
In this investigation, leaf extracts of Ocimum basilicum and Mangifira indica were used as reducing agents for biosynthesis of silver nanoparticles (AgNPs). The biosynthesized AgNPs were authorized by UV-vis spectrophotometry and X‑ray diffraction (XRD) analysis. AgNPs were obtained after 5 min of reaction at 80oC. The formation of AgNPs was confirmed by the presence of absorption peaks at 439 nm using extract from O. basilicum and at 442.5 nm from M. indica. X‑ray spectra showed strong peaks for the crystalline Ag. Shape and size of the biosynthesized AgNPs were studied using high resolution transmission electron microscope (HR-TEM). Size of the produced AgNPs was found to be 9–35 nm. Effect of the synthesized nanosilver was then investigated on some biochemical attributes of wheat plant (Triticum aestivum cultivar saka 92). The growth parameters such as shoot lengths, fresh and dry weight of shoot, chlorophyll, carbohydrate and protein contents in shoot of wheat plant were investigated. Application of AgNPs synthesized from Ocimum basilicum and from Mangifira indica at the concentrations of 20,40 ppm showed an increase in shoot length, fresh and dry weight of shoot, chlorophyll, total carbohydrate and protein content in shoot of wheat plants, beyond these concentrations an inhibitory effects were shown.
Zusammenfassung
In dieser Untersuchung wurden Blattextrakte von Ocimum basilicum und Mangifira indica als Reduktionsmittel für die Biosynthese von Silber-Nanopartikeln (AgNPs) verwendet. Die biosynthetisierten AgNPs wurden durch UV/Vis-Spektroskopie und Röntgendiffraktionsanalyse verifiziert. Die AgNPs wurden nach 5 Reaktionsminuten bei 80 °C gewonnen. Die Bildung von AgNPs wurde durch das Vorhandensein von Absorptionsspitzen bei 439 nm bei O.-basilicum-Extrakt und bei 442,5 nm bei M.-indica-Extrakt bestätigt. Röntgenspektren zeigten starke Spitzen für das kristalline Ag. Die Form und Größe der biosynthetisierten AgNPs wurden mit hochauflösender Transmissionselektronenmikroskopie (HR-TEM) untersucht. Die Größe der produzierten AgNPs lag bei 9–35 nm. Dann wurde die Auswirkung des synthetisierten Nanosilbers auf bestimmte biochemische Eigenschaften von Weizenpflanzen (Triticum aestivum, Sorte „Saka 92“) analysiert. Wachstumsparameter wie Trieblänge, Frisch- und Trockengewicht der Triebe, Chlorophyll-, Kohlenhydrat- und Proteingehalt von Weizentrieben wurden untersucht. Die Anwendung von AgNPs, synthetisiert mit Ocimum basilicum und Mangifira indica, in Konzentrationen von 20,40 ppm ergab längere Triebe, höheres Frisch- und Trockengewicht der Triebe, einen höheren Chlorophyll-, Gesamtkohlenhydrat- und Proteingehalt der Weizentriebe. Über diese Konzentrationen hinaus wurde eine inhibitorische Wirkung gezeigt.





Similar content being viewed by others
References
Abboud Y, Eddahbi A, El Bouari A, Aitenneite H, Brouzi K, Mouslim J (2013) Microwave-assisted approach for rapid and green phytosynthesis of silver nanoparticles using aqueous onion (Allium cepa) extract and their antibacterial activity. J Nanostruct Chem 3:84
Abdel-Aziz M, Shaheen MS, El-Nekeety AA, Wahhab AMA (2014) Antioxidant and antibacterial activity of silver nanopartilcels biosynthesized using Chenopodium murale leaf extract. J Saudi Chem Soc 18:356–363
Bradford MM (1976) A rapid and sensitive method or the quantitation of microgram quantities of protein using the principles of dye-binding. Anal Biochem 72:143
Chen X, Schluesener HJ (2008) Nanosilver: A nanoproduct in medical application. Toxicol Lett 176:1–12
Elgorbana AM, El-Samawatya AM, Yassina MA, Sayed SR, Adile SF, Elhindif KM, Bakrig M, Khan M (2016) Antifungal silver nanoparticles: Synthesis, characterization and biological evaluation. Biotechnol Biotechnol Equip 30(1):56–62
Elliott C (2010) The effects of silver dressings on chronic and burns wound healing. Br J Nurs 19:32–36
Farghaly FA, Nafady NA (2015) Green synthesis of silver nanoparticles using leaf extract of Rosmarinus officinalis and its effect on Tomato and Wheat plants. J Agric Sci 7(11). doi:10.5539/jas.v8n4p179
Forough M, Farhadi K (2010) Biological and green synthesis of silver nanoparticles. Turkish J Eng Env Sci 34:281–287
Ganesh Babu MM, Gunasekaran P (2013) Extracellular synthesis of crystalline silver nanoparticles and its characterization. Mater Lett 90:162–164
Gole A, Dash C, Ramakrishnaan V, Sainkar SR, Mandal AB, Rao M, Sastry M (2001) Pepsin-gold colloid conjugates: Preparation, characterization, and enzymatic activity. Langmuir 17:1674–1679
Gorinstein S, Park YS, Heo BG, Namiesnik J, Leontowicz H, Leontowicz M, Ham KS, Cho JY, Kang SG (2009) A comparative study of phenolic compounds and antioxidant and antiproliferative activities in frequently consumed raw vegetables. Eur Food Res Technol 228:903–911
Groneberg DA, Giersig M, Welte T, Pison U (2006) Nanoparticle-based diagnosis and therapy. Curr Drug Targets 7:643–648
Hedge JE, Hofreiter BT (1962) In: Whistler RL, BeMiller JN (eds) Methods in carbohydrate chemistry, vol 17. Academic Press, New York, p 420
Hojjat SS (2015) Impact of silver nanoparticles on germinated fenugreek seed. Int J Agri Crop Sci 8(4):627–630
Javanmardi J, Khalighi A, Kashi A, Bais HP, Vivanco JM (2002) Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J Agric Food Chem 50:5878–5883
Krishnaraj C, Jagan EG, Ramachandran R, Abirami SM, Mohan N, Kalaichelvan PT (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. Plant growth metabolism. Process Biochem 47:651–658
Kumar V, Yadav SK (2009) Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biot 84:151–157
Liu XM, Zhang FD, Zhang SQ, He XS, Fang R, Feng Z, Wang Y (2005) Effects of nano – ferric oxide on the growth and nutrients absorption of peanut. Plant Nutr Fert Sci 11:14–18
Liyana-Pathirana CM, Shahidi F (2005) Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J Agric Food Chem 53:2433–2440
Logeswari P, Silambarasan S, Abraham J (2015) Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J Saudi Chem Soc 19:311–317
Lu X, Wang J, Al-Qadiri HM, Ross CF, Powers JR, Tang J, Rasco BA (2011) Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem 129:637–644
Mansoori AG (2005) “Advances in atomic and molecular nanotechnology,” Principles of nanotechnology, molecular-based study of condensed matter in small systems, pp 1–30 (chapter 1)
Metzner H, Rau H, Senger H (1965) Untersuchungen Zur Synchronisierbarkeit einzelner Pigment-Mangel Mutanten von Chlorella. Planta 65:186
Mori Y, Ono T, Miyahira Y, Quang V, Nguyen VQ, Matsui T, Ishihara M (2013) Antiviral activity of silvernanoparticle/chitosan composites against H1N1 influenza. A virus. Nanoscale Res Lett 8:93
Muknthan K, Balaji S (2012) Cashew apple juice (Anacardium occidentale L.) speeds u up the synthesis of silver nanoparticles. Internat J Green Nanotechnol 4:71–79
Najafi S, Heidari R, Jamei R (2014) Photosynthetic characteristics, membrane lipid levels and protein content in the Phaseolus vulgaris L. (cv. Sadri) exposed to magnetic field and silver nanoparticles. Bull Environ Pharmacol Life Sci 3:72–76
Racuciu M, Creanga DE (2007) TMA-OH Coated magnetic nanoparticles internalized in vegetal tissue. Roman J Phys 52:395–402
Salama HMH (2012) Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int Res J Biotechnol 3:190–197
Saxena A, Tripathi RM, Singh RP (2010) Biological synthesis of silver nanoparticles by using onion (Allium cepa) extract and their antibacterial activity. Dig J Nanomat Biostruct 5(2):427–432
Saxena A, Tripathi RM, Zafar F, Singh P (2012) Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater Lett 67:91–94
Senapati S, Ahmad A, Khan MI, Sastry M, Kumar R (2005) Extracellular biosynthesis of bimetallic au-ag alloy nanoparticles. Small 1(5):517–520
Shah KA, Patel MB, Patel RJ, Parmar PK (2010) Mangifera Indica (Mango). Pharmacogn Rev 4(7):42–48
Singhal G, Bhavesh R, Kasariya K, Sharma AR, Singh RP (2011) Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nanopart Res 13:2981–2988
Statistical Analysis System (2006) SAS user’s guide: Statistics. SAS Institute Inc.Editors, Cary, NC
Sharma P, Bhatt D, Zaidi MG, Saradhi PP, Khanna PK, Arora S (2012) Silver nanoparticlemediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol 167:2225–2233
Acknowledgements
The authors are thankful to Dr. Mona Mostafa Saif Assistant professor of inorganic chemistry, Faculty of Education, Ain Shams University, for helping us with UV-Vis spectroscopy and FTIR analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Latif, H.H., Ghareib, M. & Tahon, M.A. Phytosynthesis of Silver Nanoparticles using Leaf Extracts from Ocimum basilicum and Mangifira indica and their Effect on some Biochemical Attributes of Triticum aestivum . Gesunde Pflanzen 69, 39–46 (2017). https://doi.org/10.1007/s10343-017-0385-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-017-0385-9
Keywords
- Green synthesis
- Silver nanoparticles
- TEM (Transmission Electron Microscopy)
- Chlorophyll
- Carbohydrate
- Protein