Abstract
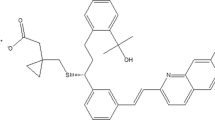
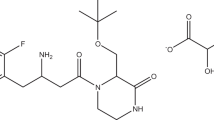
Analytical quality by design (AQbD)-oriented liquid chromatographic method development for determination of telmisartan and its impurities A, C, and 1 is determination is presented. Step-by-step process was conducted in order to define reliable design space. At the beginning, critical process parameters with the highest influence on method performance were defined: acetonitrile content in the first (ACN 1) and second (ACN 2) gradient step and time (t 2) the second gradient step. These factors were varied according to Box–Behnken plan of experiments and their influence on retention times of impurities A and C, S value between telmisartan and impurity 1 and peak capacity were followed. In this way, the relationship between the critical process parameters and critical quality attributes was established. The obtained mathematical models and Monte Carlo simulations were used to identify the design space. Fractional factorial design was applied for experimental robustness testing, and the method was validated to verify the adequacy of selected optimal conditions. Finally, all validation parameters were tested, and adequacy of the method was confirmed. Applicability as a routine method was confirmed by analysis of commercially available tablets.






Similar content being viewed by others
References
Department of Health and Human Services, U.S. Food and Drug Administration (2007) Pharmaceutical quality for 21st Century a risk based approach progress report. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm128080.htm. Accessed 8 Feb 2017
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline (2009) Pharmaceutical development Q8 (R2). ICH, Geneva
International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline (2005) Quality risk management Q9. ICH, Geneva
International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline (2008) Pharmaceutical quality system Q10. ICH, Geneva
Rozet E, Lebrun P, Debrus B, Boulanger P, Hubert P (2013) Design spaces for analytical methods. Trac-Trend Anal Chem 42:157–167
Orlandini S, Pinzauti S, Furulanetto S (2013) Application of quality by design to the development of analytical separation methods. Anal Bioanal Chem 405:443–450
Jančić-Stojanović B, Rakić T (2015) In: Anderson J, Berthod A, Pino V, Stalcup AM (eds) Analytical separation science. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Nistor I, Lebrun P, Ceccato A, Lecomte F, Slama I, Oprean R, Badarau E, Dufour F, Sylvestre Dossou KS, Fillet M, Liégeois J-F, Hubert Ph, Rozet E (2013) Implementation of a design space approach for enantiomeric separations in polar organic solvent chromatography. J Pharmaceut Biomed 74:273–283
Molnár I, Rieger H-J, Monks KE (2010) Aspects of the “design space” in high pressure liquid chromatography method development. J Chromatogr A 1217:3193–3200
Monks KE, Rieger H-J, Molnár I (2011) Expanding the term “design space” in high performance liquid chromatography (I). J Pharmaceut Biomed 56:874–879
Monks K, Molnár I, Rieger H-J, Bogáti B, Szabó E (2012) Quality by design: multidimensional exploration of the design space in high performance liquid chromatography method development for better robustness before validation. J Chromatogr A 1232:218–230
Schmidt AH, Molnár I (2013) Using an innovative Quality-by-design approach for development of a stability indicating UHPLC method for ebastine in the API and pharmaceutical formulations. J Pharmaceut Biomed 78–79:65–74
Tumpa A, Miladinović T, Rakić T, Stajić A, Jančić-Stojanović B (2016) Quality by design determination of diclofenac potassium and its impurities by high-performance liquid chromatography. Anal Lett 49:445–457
Mallik R, Raman S, Liang X, Grobin AW, Choudhury D (2015) Development and validation of a rapid ultra-high performance liquid chromatography method for the assay of benzalkonium chloride using a quality-by-design approach. J Chromatogr A 1413:22–32
Tumpa A, Stajić A, Jančić-Stojanović B, Medenica M (2017) Quality by design in the development of hydrophilic interaction liquid chromatography method with gradient elution for the analysis of olanzapine. J Pharmaceut Biomed 134:15–26
Debrus B, Guillarme D, Rudaz S (2013) Improved quality-by-design compliant methodology for method development in reversed-phase liquid chromatography. J Pharmaceut Biomed 84:215–223
Ruth RW, William JC, John DI, Michael RC, Kristine P, Ronald DS, Pieter BMWMT (1996) Nonpeptide angiotensin II receptor antagonists: the next generation in antihypertensive therapy. J Med Chem 39:625–656
European Pharmacopoeia (2016) 8th edn. Council of Europe, Strasbourg, pp 3369–3370
Ries UJ, Mihm G, Narr B, Hasselbach KM, Wittneben H, Entzeroth M, van Meel JCA, Wienen W, Hauel NH (1993) 6-substituted benzimidazoles as new nonpeptide angiotensin II receptor antagonists: synthesis, biological activity, and structure-activity relationships. J Med Chem 36:4040–4051
Sanjeev Kumar A, Ghosh S, Mehta GN, Soundararajan R, Sarma PSR, Bhima K (2009) Efficient and improved synthesis of telmisartan: an antihypertensive drug. Synthetic commun 39:4149–4157
Lakkoju C, Koneti NR, Kokkalla S, Mallela SPS, Boyapati NC (2012) An improved process for the preparation of telmisartan. WO 2012/028925 A2
Charde MS, Gupta A, Chakole RD (2012) Determination of telmisartan in pharmaceutical formulation by reverse phase-high performance liquid chromatography. Int J Phytopharm 2:61–67
Dhanawade PP, Kane RN, Patrekar PV, Mali SS (2014) A Validated RP-HPLC method for the determination of telmisartan in bulk and pharmaceutical dosage form. Int J Pharm Adv Anal 4:126–129
Suresh Kumar GV, Rajendraprasad Y (2010) Development and validation of revered-phase HPLC method for simultaneous estimation of telmisartan and amlodipin in tablet dosage form. Int J Pharm Pharm Sci 3:128–131
Mhaske RA, Garole DJ, Mhaske AA, Sahasrabudhe S (2012) RP-HPLC method for simultataneous determination of amlodipine besylate, valsartan, telmisartan, hydrochlorothiazide and chlorthalidone: application to commercially available drug products. Inter J Pharm Sci Res 3:141–149
Chitra P, Ganesa SS, Arumugam K, Suvarna K, Mallayasamy SR, Nayanabhirama U (2007) Determination of telmisartan by HPTLC-A stability indicating assay. JPC J Planar Chromat 20:477–481
Nageswara Rao R, Guru Prasad K, Gangu Naidu Ch, Maurya PK (2011) Development of a validated liquid chromatographic method for determination of related substances of telmisartan in bulk drug and formulation. J Pharmaceut Biomed 56:471–478
Ferreiros N, Iriarte G, Alonso RM, Jimenez RM, Ortiz E (2006) Separation and quantitation of several angiotensin II receptor antagonist drugs in human urine by a SPE–HPLC–DAD method. J Sep Sci 29:650–655
Zhang H, Jiang YY, Wen J, Zhou TT, Fan GR, Wu YT (2009) Rapid determination of telmisartan in human plasma by HPLC using a monolithic column with fluorescence detection and its application to a bioequivalence study. J Chromatogr B 877:3729–3733
Brunetto MR, Contreras Y, Clavijo S, Torres D, Delgado Y, Ovalles F, Ayala C, Gallignani M, Estela JM, Martin VC (2009) Determination of losartan, telmisartan, and valsartan by direct injection of human urine into a column-switching liquid chromatographic system with fluorescence detection. J Pharmaceut Biomed 50:194–199
Torrealday N, Gonzalez L, Alonso RM, Jimenez RM, Ortiz Lastra E (2003) Experimental design approach for the optimization of a HPLC-fluorimetric method for the quantitation of the angiotensin II receptor antagonist telmisartan in urine. J Pharmaceut Biomed 32:847–857
B-m Chen, Y-z Liang, Y-l Wang, Deng FL, Zhou P, F-q Guo, L-f Huang (2005) Development and validation of liquid chromatography–mass spectrometry method for the determination of telmisartan in human plasma. Anal Chim Acta 540:367–373
Yan T, Li H, Deng L, Guo Y, Yu W, Fawcett JP, Zhang D, Cui Y, Gu J (2008) Liquid chromatographic–tandem mass spectrometric method for the simultaneous quantitation of telmisartan and hydrochlorothiazide in human plasma. J Pharmaceut Biomed 48:1225–1229
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline (2005) Validation of analytical procedures: text and methodology Q2(R1). ICH, Geneva
Crowther J (2001) In: Ahuja S, Scypinsky S (eds) Handbook of modern pharmaceutical analysis. Academic Press, San Diego
MarvinSketch 15.1.26 (2015) ChemAxon, Budapest. http://www.chemaxon.com. Accessed 8 Feb 2017
Mbinze JK, Lebrun P, Debrus B, Dispas A, Kalenda N, Mavar Tayey Mbay J, Schofield T, Boulanger B, Rozet E, Hubert Ph, Marini RD (2012) Application of an innovative design space optimization strategy to the development of liquid chromatographic methods to combat potentially counterfeit nonsteroidal anti-inflammatory drugs. J Chromatogr A 1263:113–124
Jančić-Stojanović B, Rakić T, Malenović A (2013) In: Ramos F (ed) Liquid chromatography: principles, technology and applications. NOVA Science Publishers, New York
Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, Layloff T, Viswanathan CT, Edgar Cook C, McDowall RD, Pittman KA, Spector S (1992) Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Pharm Res 9:588–592
Acknowledgements
This work was financially supported by the Ministry of Education, Science and Technological Development, Republic of Serbia, as part of Project no. 172041.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Ministry of Education, Science and Technological Development, Republic of Serbia (Grant no. 172041).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dobričić, V., Vukadinović, D., Jančić-Stojanović, B. et al. AQbD-Oriented Development of a New LC Method for Simultaneous Determination of Telmisartan and Its Impurities. Chromatographia 80, 1199–1209 (2017). https://doi.org/10.1007/s10337-017-3330-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-017-3330-2