Abstract
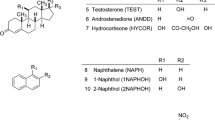
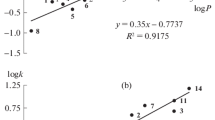
The aim of the study was to characterise the unique chromatographic properties of tetrahydrofuran (THF) based on hydrogen-bonding interactions with weakly acidic compounds (pK a = 7.4–12.48) including steroids with phenolic hydroxyl groups, their substituted derivatives and heterocyclic amides having different polar functional groups (log P = 1.15–4.78). The results suggested that the organic modifier does not simply affect retention by changing the hydrophobicity of eluent, but rather specifically modifies the nature of the analyte–stationary phase interaction. In the water/isopropanol (IPA)/THF eluent mixture THF forms a hydrogen-bonded complex with the phenolic steroid compounds. The apparent formation of the THF–analyte complex depended on the proportion of components in the ternary mobile phase employed (from 70:30:0 to 70:0:30 v/v/v). The weakly acidic model compounds showed an increasing retention time with increasing THF concentration. This effect of THF was found to be a solvent-specific interaction, which was only observed in the presence of IPA. The systematic modification of the phase ratio of organic modifiers exerts a great influence on retention time and changes the separation processes over a wide range. In the case of other protic solvents (methanol, ethanol) we could not observe this selective chromatographic behaviour. From the point of view of chromatographic practice, the use of THF–IPA co-modifiers may increase the selectivity and provide excellent possibilities for separation of weakly acidic compounds including the large family of phenolic compounds.

Similar content being viewed by others
References
Rafferty JL, Stepmann JI, Schure MR et al (2009) Adv Chromatogr 48:1–55
Gritti F, Guiochon G (2005) Anal Chem 77:4257–4272
Gritti F, Guiochon G (2012) J Chromatogr A 1228:2–19
Rafferty L, Zhang L, Siepmann JI, Schure MR (2007) Anal Chem 79:6551–6558
Cheong WJ, Carr PW (1988) Anal Chem 60(8):820–826
Rutan SC, Carr PW, Cheong WJ, Park JH, Snyder LR (1989) J Chromatogr 463:21–37
Miyabe K, Sotoura S, Guiochon G et al (2001) J Chromatogr A 919:231–244
Barbosa J, Barrón D, Buti S (1999) Anal Chim Acta 389:31–42
Roggendorf E, Spatz R (1981) J Chromatogr 204:263–268
Camoes MF, Guiomar Lito MJ, Ferra MIA, Covington AK et al (1997) Pure Appl Chem 69:1325–1334
Lu H, Rutan SC (1996) Anal Chem 68:1387–1393
Lu H, Rutan SC (1999) Anal Chim Acta 388:345–352
Marcus Y (1998) The properties of solvents. Wiley, New York
Reichardt C, Welton T (2011) Solvents and solvent effects in organic chemistry. Wiley, New York
Soczewinsky E, Waksmundzka-hajnos M (1980) J Liq Chromatogr 3(11):1625–1636
Knox H, Pryde A (1975) J Chromatogr 112:171–188
Kazakevich YV, LoBrutto R, Chan F, Patel T (2001) J Chromatogr A 913:75–87
LoBrutto R, Kazakevich Y (2007) HPLC for pharmaceutical scientist. Wiley, Indianapolis
Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Fryi RF (1999) J Clin Pharmacol 39:327–348
Marwah A, Marwah P, Lardy H (2001) J Chromatogr A 935:279–296
Kazihnitkova H, Tejkalova H, Benesova O, Bicikova M, Hill M, Hampl R (2004) Steroids 69:667–674
Marwah A, Marwah P, Lardy H (2002) J Chromatogr B 767:285–299
Gergely A, Gy Szász, Szentesi A, Gyimesi-Forrás K, Kökösi J, Szegvári D, Veress G et al (2006) Anal Bioanal Chem 384:1506–1510
Gergely A, Horváth P, Szász Gy, Veress G et al (2009) Anal Bioanal Chem 394:2105–2109
Riddick JA, Bumger WB (1970) Organic solvents. Wiley, New York
Kökösi J, Őrfi L, Szász G, Hermecz I, Kapui Z, Szabó M (1992) Hungarian Patent HU59411
Váradi A, Horváth P, Kurtán T, Mándi A, Tóth G, Gergely A, Kökösi J (2012) Tetrahedron 68:10365–10371
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horváth, P., Gergely, A., Mazák, K. et al. Novel Data on the Effect of Tetrahydrofuran as an Organic Co-Modifier in RP-HPLC. Chromatographia 76, 441–448 (2013). https://doi.org/10.1007/s10337-013-2421-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-013-2421-y