Abstract
Background
Although some antiseizure medications (ASMs) are teratogenic, most people with epilepsy need treatment in pregnancy. The risk of ASM fetotoxicity may be mitigated with folic acid. High-dose folic acid supplementation has traditionally been recommended before and during gestation despite little evidence of efficacy and safety for this patient group. Several studies have investigated the potential benefits and risks of folic acid supplements.
Objective
To provide an updated overview of the risks, benefits, and rationale for use of folic acid supplementation in relation to pregnant people of childbearing age using ASM.
Materials and methods
This is a narrative review based on an unstructured literature search of PubMed. We also scrutinized neurological and obstetrical guidelines.
Results
Antiseizure medication can decrease folate concentrations. In children exposed to ASM prenatally, those born to persons using folic acid supplements periconceptionally had lower risk of adverse neurodevelopment and preterm birth. It remains unclear whether the risk for congenital malformations can be equally alleviated. In studies of the general population, high plasma folate concentrations and/or high-dose folic acid supplements were associated with adverse neurodevelopmental outcomes. This has not been seen in children of mothers with epilepsy. However, an increased cancer risk has been found in children of mothers with epilepsy using high-dose folic acid supplements in pregnancy.
Conclusion
The optimal folic acid dose is not clear for persons of childbearing potential with epilepsy using ASM. Both low and excess folate status during pregnancy have been associated with adverse neurodevelopment. We propose an individual folic acid supplement dose that should be titrated based on maternal plasma folate concentrations during pregnancy.
Zusammenfassung
Hintergrund
Obwohl einige Antiepileptika („antiseizure medications“, ASM) embryotoxisches Potenzial aufweisen, sind sie für die meisten Menschen mit Epilepsie in der Schwangerschaft unentbehrlich. Das Risiko einer ASM-Embryotoxizität kann mit Folsäure gemindert werden. Die Einnahme von hochdosierter Folsäure wird traditionell vor und während der Schwangerschaft empfohlen, obwohl nur wenige Beweise für deren Wirksamkeit und Sicherheit für diese Patientengruppe vorliegen. In den letzten Jahren haben mehrere Studien die möglichen Vorteile und Risiken von Folsäurepräparaten untersucht.
Zielsetzung
Das Anliegen der Autoren ist es, einen aktualisierten Überblick über die Beweggründe für die Anwendung, Risiken und Vorteile der Einnahme von Folsäure zu geben im Hinblick auf eine Schwangerschaft bei Personen im gebärfähigen Alter, die mit Antiepileptika behandelt werden.
Materiale und Methoden
Diese narrative Übersicht stützt sich auf eine unstrukturierte Literatursuche bei PubMed. Neurologische und geburtshilfliche Richtlinien wurden zusätzlich konsultiert.
Ergebnisse
Antiepileptika können die Folsäurekonzentration im Plasma senken. Kinder, die während der Schwangerschaft gegenüber Antiepileptika exponiert wurden und die von Personen geboren wurden, die perikonzeptionell Folsäurepräparate eingenommen hatten, wiesen ein geringeres Risiko für eine unerwünschte neurologische Entwicklung und eine Frühgeburt auf. Ob das Risiko für angeborene Fehlbildungen ebenso gemindert werden kann, ist bisher nicht geklärt. Studien in der Allgemeinbevölkerung zeigten, dass hohe Folsäurekonzentrationen im Plasma und/oder die Einnahme hochdosierter Folsäurepräparate mit negativen Auswirkungen auf die neurologische Entwicklung der Kinder in Verbindung gebracht wurden. Dies wurde allerdings bei Kindern von Schwangeren mit Epilepsie nicht beobachtet. Es wurde jedoch ein erhöhtes Krebsrisiko bei Kindern von Frauen mit Epilepsie festgestellt, die in der Schwangerschaft hochdosierte Folsäurepräparate eingenommen hatten.
Schlussfolgerung
Die optimale Folsäuredosis ist für Personen mit Epilepsie im gebärfähigen Alter, die Antiepileptika einnehmen, nicht geklärt. Sowohl zu niedrige als auch sehr hohe Folsäurekonzentrationen während der Schwangerschaft wurden mit einer nachteiligen Entwicklung der Neuronen in Verbindung gebracht. Die Autoren schlagen einen individuell angepassten Ansatz vor, bei dem sich die Höhe der Folsäuredosis nach der mütterlichen Folsäurekonzentration im Plasma während der Schwangerschaft richtet.
Similar content being viewed by others
Introduction
Most pregnant persons with epilepsy need antiseizure medication (ASM) throughout their pregnancy to prevent epileptic seizures [1]. However, ASM may be associated with adverse pregnancy outcomes such as spontaneous abortion, fetal growth restriction, preterm birth, congenital malformations, or adverse neurodevelopment in the child [1]. To mitigate fetotoxicity, most clinical guidelines recommend high-dose folic acid supplements (≥ 1 mg daily) in conjunction with ASM before and during pregnancy [2] (Tab. 1).
Folate is critical for normal fetal growth and development, for synthesis and repair of DNA and RNA, for gene methylation, and for the metabolism of amino acids [3]. While some ASMs interact with folic acid metabolism and potentially reduce folate levels [4], the benefit and harm of high-dose folic acid and potential excessive folate in people of childbearing potential using ASM are unclear. Despite the widespread recommendation of folic acid supplementation for this patient group before and during pregnancy, there is no consensus regarding the optimal dose, start, or duration of supplementation. Current recommendations vary from 0.4 mg to 5 mg daily during the periconceptional period or during the entire pregnancy [5,6,7,8,9,10]. This variability leads to discrepancies in clinical practice and can cause confusion and uncertainty among healthcare providers and patients. Since individual folate concentrations vary with different medical conditions, genetic makeup, socioeconomic status, race/ethnicity, and intake of folic acid in non-fortified and fortified foods [11], it is also possible that a “one dose fits all” is not a reasonable strategy.
The aim of this review is to provide an updated overview of the interplay between folate, ASM, and pregnancy, addressing the evidence on the benefit and harm of supplementation.
Material and methods
This is a narrative review based on an unstructured literature search in PubMed with the following keywords and MeSH terms: (folate OR “Folic Acid”[MeSH]) AND (“Epilepsy”[MeSH] OR epileptic OR antiepileptic OR antiseizure OR “Anticonvulsants”[MeSH]) AND (women OR “Pregnancy”[MeSH] OR female*). The bibliography of each relevant article was scrutinized for additional citations. We included studies in English or Scandinavian languages available until March 2023. We consulted the epilepsy treatment guidelines from the American Academy of Neurology, the International League Against Epilepsy (ILAE), the European Academy of Neurology (EAN, previously EFNS), UpToDate, the Royal College of Obstetricians and Gynaecologists, the National Institute for Health and Care Excellence (NICE), and other guidelines from various countries.
Results
What determines serum folate concentration?
Folate, also known as vitamin B9, is a naturally occurring essential nutrient found in a variety of foods, including vegetables, fruits, seafood, eggs, dairy products, meat, and grains. Folic acid on the other hand, represents the synthetic and biochemically more stable form of folate, used in supplements and fortified foods. It is converted to folate upon absorption. The nutrient enters the folate cycle and is converted to the metabolically active 5‑methyltetrahydrofolate (MTHF). In conjunction with cobalamin (vitamin B12), MTHF is necessary for the remethylation of homocysteine to produce methionine, an important methyl donor. Without MTHF, cobalamin, riboflavin (vitamin B2), and pyridoxine (vitamin B6), homocysteine concentrations increase. Serum folate (MTHF) is the primary marker of folate status. Food intake before blood sampling may affect folate concentrations. Red blood cell folate is considered a better indicator of body stores and nutritional status, but the reliability of the analytical methods is questioned, and many laboratories no longer offer this analysis. Homocysteine is the metabolic marker of folate status. Homocysteine concentrations start to rise when serum folate falls below 25–27 nmol/L, indicating an insufficient intracellular folate status [12]. Folate concentrations depend on the intake of folate through the diet, on the intake of folic acid in fortified food and in supplements, as well as on individual factors such as body mass index, socioeconomic factors, country, and race/ethnicity [11]. When folic acid intake is excessive, unmetabolized folic acid (UMFA) can accumulate in plasma. Genetic risk factors also determine folate status. These are common and include single-nucleotide polymorphisms (SNPs) in genes regulating the one-carbon metabolism. Such SNPs may disturb vitamin B uptake, transport, and enzymatic activity, causing an increased demand for folate intake [13]. The most important SNP is the 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C→T genotype, which reduces enzyme activity by 50% in homozygous persons and causes low folate concentrations and homocysteinemia [13].
Importance of folate in pregnancy
Folate demand increases during pregnancy due to the rapid growth of the fetus, the placenta, and the maternal tissue. Folate deficiency during pregnancy is associated with maternal anemia as well as poor implantation and vascularization of the placenta and subsequently with spontaneous abortions, preterm birth, preeclampsia, fetal growth restriction, and other placenta-related pregnancy complications [3]. Folate deficiency is also associated with congenital malformations such as neural tube and cardiac defects as well as neurodevelopmental disorders such as autism spectrum disorder [14]. The exact mechanisms involved are largely unknown, but suggested mechanisms are alterations in DNA and RNA synthesis, accumulation of toxic concentrations of homocysteine, and altered gene methylation [3].
In the general population, periconceptional folic acid supplementation prevents neural tube defects [3]. Therefore, the World Health Organization (WHO) recommends all persons planning pregnancy to take 0.4 mg folic acid supplements daily preconceptionally and throughout pregnancy. Recent studies reported that periconceptional folic acid supplementation was associated with a decreased risk of adverse neurodevelopment in the children, such as autism spectrum disorders and language impairment, and with improved cognitive performance [15]. As unplanned pregnancies are common and adherence to the supplement guidelines low, in many countries—including the United States (US) and since 2021 the United Kingdom—it is mandatory to fortify certain foods with folic acid [16]. After initiating this program, the frequency of neural tube defects decreased in the US [11]. Most other Western and Nordic European countries do not fortify foods with folic acid. However, since the individual folate need depends on many factors, supplementation according to the serum folate concentration is emphasized by guidelines for persons with childbearing potential in the general population and is recommended by the WHO [11]. The recommendations are based on high-quality data regarding the association between folate concentrations and neural tube defects. Serum folate concentrations of > 28–30 nmol/L or red blood cell folate concentrations > 906 nmol/L have been extensively evaluated in pregnant populations, and there is clear evidence that maternal red blood cell folate concentrations > 906 nmol/L protect against folic acid-related neural tube defects in the fetus [15]. Persons from the general population may reach a preventive red blood cell folate concentration of more than 906 nmol/L within 4 weeks of supplementation with a daily intake of 800 µg folic acid [17]. The prevalence of having a red blood cell folate concentration of < 906 nmol/L was 35% after 40 weeks with a daily folic acid supplement of 140 µg and 18% with 400 µg [18].
Folate and antiseizure medication
Some ASMs interact with the uptake and metabolism of folate, especially those that induce cytochrome P450 enzymes [19]. Carbamazepine, phenobarbital, phenytoin, and primidone increase folate catabolism, which in turn may impede the remethylation of homocysteine to methionine, thereby increasing homocysteine concentrations. Low folate or high homocysteine concentrations have been reported after chronic use of valproate, topiramate, gabapentin, oxcarbazepine, and levetiracetam while there are fewer data for lamotrigine [19].
The few studies on folate among pregnant persons with epilepsy have uncovered that individuals using lamotrigine had lower folate metabolite concentrations compared to untreated persons with epilepsy [20]. High ASM concentrations have been associated with high concentrations of UMFA and inactive folate metabolites, and with a low ratio between MTHF and its inactive metabolites, indicating increased folate catabolism [4].
Valproate in particular has been associated with impaired folate absorption and metabolism, accumulation of homocysteine, impaired DNA methylation, inhibition of folate receptors and carriers, and with low brain and placental folate concentrations [21]. In zebrafish embryos, folic acid supplementation reduced valproate-induced structural brain defects and neurotoxicity [22]. Regarding other ASMs, folic acid supplementation protected against lamotrigine-induced offspring malformations in mice [23]. In human placentas, lacosamide downregulated folate carriers potentially affecting folate supply to the fetus [24]. In human embryonic stem cells exposed to carbamazepine, gabapentin, lamotrigine, levetiracetam, or topiramate, all ASMs were associated with DNA damage, particularly levetiracetam and topiramate, whereas folic acid decreased this DNA damage [25].
Folic acid supplements could thus potentially reduce the risk of ASM toxicity in pregnancy [21, 26]. As many of the adverse outcomes in children seen after maternal folate deficiency in the general population overlap with those seen after prenatal ASM exposure, authors have suggested that some of the adverse effects of ASM in the fetus could be mediated through folate deficiency. Children in the prospective Norwegian Mother, Father and Child (MoBa) cohort study prenatally exposed to ASM had a threefold higher risk of preterm birth if the mother did not use periconceptional folic acid compared with children of mothers who did [27]. If persons with epilepsy were untreated during pregnancy, the risk of preterm birth was not modified by folic acid (Fig. 1; [27]). On the other hand, periconceptional folic acid supplementation has not convincingly been shown to reduce the risk of ASM-associated congenital malformations, in contrast to findings in the general population [28,29,30,31,32]. Data from the International Registry of Antiepileptic Drugs and Pregnancy (EURAP) have even indicated an increased risk of congenital malformations after folic acid supplementation [29]. There are conflicting results as to whether supplementation with folic acid is associated with a reduced risk of spontaneous abortions [33, 34]. However, in people using ASM, plasma folate and red blood cell folate concentrations were significantly lower prior to pregnancy in those who had a spontaneous abortion or a child with congenital anomalies compared to persons with a healthy pregnancy outcome [35]. Also, children born to mothers carrying the MTHFR 677TT mutation had a higher risk of congenital anomalies compared to those carrying the MTHFR 677CC wildtype alleles [36].
Proportion (%) of persons with antiseizure medication-treated epilepsy (a), persons with untreated epilepsy (b), and persons without epilepsy (c) using folic acid supplementation at different time points during pregnancy. Red lines indicate people with preterm delivery; orange lines indicate people without preterm delivery. Preterm birth: gestational age < 37 weeks. *p < 0.05. (From Alvestad et al. [27])
The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study found that periconceptional folic acid supplement use was associated with improved child IQ scores in ASM-exposed children of persons with epilepsy [37, 38]. At the age of 6 years, the mean IQ for children exposed to periconceptional folate supplementation was 108 (95% confidence interval [CI]: 106–111) compared with 101 (95% CI: 98–104) for children not exposed to folic acid (Fig. 2; [37]). This association was also present in sub-analyses of children exposed to lamotrigine. However, maternal dietary folate intake did not affect the child outcome [39]. In some studies [40,41,42,43], but not all [44,45,46], it was found that children prenatally exposed to ASM also had a higher risk for autistic traits or language impairment if the mother reported no use of periconceptional folic acid compared to children of supplement users. Similar associations were not seen for women with epilepsy not using ASM [40,41,42].
Child IQ at 6 years, by exposure to maternal antiepileptic drug use and periconceptional folate supplementation. Means (95% confidence intervals) are shown for folate (solid lines) and no folate (dashed lines) supplementation. (From Meador et al. 2013 [37], reused with permission from Elevier)
Adverse effects of excess folic acid
Many clinical guidelines recommend high doses of folic acid (1–5 mg) during pregnancy for persons who are using ASM, as well as for those who previously had children with congenital malformations, who smoke, and who have diabetes, obesity, or inflammatory bowel disease, in order to prevent congenital malformations [2]. However, there are concerns that high-dose folic acid and UMFA can be harmful for the mother [47]. Some studies have indicated that the use of high-dose folic acid promotes cancer growth and increases DNA de novo point mutations [48, 49], although other studies have shown a protective or null effect on cancer development [50]. Studies of pregnant people have not shown an increased risk of cancer among those taking regular doses (0.4–0.8 mg) of folic acid supplementation [51]. However, there are still conflicting results regarding the effect of folic acid in higher doses and as to whether there is a threshold for the dose, duration, or timing of folate supplementation that could potentially increase the risk of cancer [47, 52].
Folic acid intake can mask vitamin B12 deficiency, and vitamin B12 concentrations should therefore be routinely checked before starting folic acid supplementation [52]. Vitamin B12 deficiency should be treated in conjunction with folic acid supplementation.
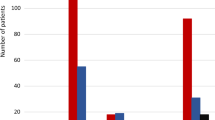
It has been suggested that high maternal prenatal folate concentrations could harm the child. A recent observational cohort study including all children in Scandinavia found an increased risk of cancer in children of mothers with epilepsy filling prescriptions for high-dose folic acid supplements (1 mg or more) during pregnancy. Children of high-dose folic acid users without epilepsy and children of mothers using ASM, but not high-dose folic acid, had no increased risk of cancer (Fig. 3; [53]). In pregnant persons without epilepsy and in mice, excess folic acid intake and high folate concentrations have also been associated with increased risk of adverse neurodevelopment in the offspring [54, 55]. In humans, high doses of folic acid during pregnancy and high UMFA concentrations in the umbilical cord blood were associated with increased risk of child autism spectrum disorder [56,57,58], and with impaired psychomotor development in children at 1 year of age [59]. However, in children of persons treated with ASM and folic acid in pregnancy, UMFA was detected but not related to adverse neurodevelopment in the children [60].
Cumulative incidence of childhood cancer with or without maternal prescription filled for high-dose folic acid for mothers with (a) or without (b) epilepsy. (From Vegrim et al. 2022 [53])
Folic acid dose
For individuals using ASM, the optimal folic acid supplement dose before, during, and after pregnancy is not known. There is no consensus on the timing and dose of folic acid, with clinical guidelines varying internationally (Table 1).
In an effort to assess how many clinicians adhere to clinical guidelines, the ILAE conducted a global survey showing that 52 of the 57 responding ILAE chapters had guidelines that included folate recommendations [10]. Most respondents stated that their guidelines recommended ≥ 4 mg folic acid daily, but ranging from 0.4 mg to ≥ 4 mg [10]. Besides supplementing folic acid in relation to pregnancy, some guidelines, including the ILAE as well as Norwegian guidelines, recommend 0.4 mg folic acid daily to all individuals treated with ASM of childbearing age, regardless of their pregnancy plans, due to the high prevalence of unplanned pregnancies in persons with epilepsy [61, 67, 68].
There are no current prospective studies designed to investigate different folic acid doses in people using ASM. The high-dose folic acid recommendations are largely based on a study that randomized pregnant persons who previously had given birth to a child with a congenital malformation to receive either 4 mg folic acid, no supplement, or a multivitamin supplement without folic acid. The results favored folic acid, but the study was not designed to investigate high-dose vs. low-dose supplementation [69] Another multicenter, double-blind randomized controlled trial of 1060 women planning a pregnancy reported that supplementation with 4.0 mg vs. 0.4 mg of folic acid was not associated with reduced occurrence of congenital malformations, but was associated with a lower risk of other adverse pregnancy outcomes such as spontaneous abortion, small for gestational age, and preterm delivery [15]. However, these studies did not specifically examine persons with epilepsy. In the NEAD study that showed an association between folic acid and improved IQ scores in children prenatally exposed to ASM, most of the women used more than 1 mg folic acid, and a dose-dependent effect was found [37]. In the Norwegian MoBa study, the plasma folate concentration in pregnant individuals with epilepsy in gestational week 18 was inversely associated with autistic traits (Fig. 4), but not with language impairment [40, 41] in children prenatally exposed to ASM. The patient-reported folic acid doses were also associated with the degree of autistic symptoms (Fig. 4; [40]).
Degree of autistic traits at 36 months of age according to folic acid dose used before and during pregnancy. Plot: mean autistic traits score and corresponding 95% error bars. Adjusted (Adj) beta: the standardized regression coefficient from a linear regression model adjusted for parity, socioeconomic factors, antiepileptic drug serum level, maternal smoking, and number of generalized tonic clonic seizures during pregnancy and valproate sodium use. (Reproduced with permission from Bjørk et al. [40]. Copyright©(2018) American Medical Association. All rights reserved)
In the absence of clear evidence on which folic acid dose is optimal to maximize potential benefits but also to avoid harm, we previously suggested a supplement of at least 0.4 mg daily for people of childbearing potential using ASM, but keeping the folic acid doses at ≤ 4 mg daily [70].
Discussion
Children exposed to ASM prenatally are less often born preterm and have a lower risk of neurodevelopmental disorders if folic acid supplements were used in pregnancy, according to some but not all studies. However, in contrast to preclinical data and data from the general population, no studies have demonstrated that folic acid supplementation protects against ASM-related congenital malformations. The divergent results between studies can be explained by the difficulty in separating the effect of the folic acid supplement-demanding maternal condition itself from the effect of folate on child outcome. As pregnant persons using valproate, ASM polytherapy, and high ASM doses often take high doses of folic acid, non-folate-related teratogenicity may overshadow the beneficial effects of folic acid supplementation. On the other hand, adherence to folic acid supplementation is related to socioeconomic status and failure to adjust for such confounding factors may bias results in favor of folic acid [69]. Further, as the food fortification practices and recommendations for folic acid supplementation vary greatly, the de facto folate status has most likely been very different within the study groups and between the studies. It is also important to mention that most studies were not designed to examine associations between periconceptional folic acid exposure and ASM-related adverse outcome, and therefore had not recorded detailed prospective data on dose, timing, or adherence to folic acid supplementation; furthermore, the majority of studies had not included folate or homocysteine measurements [29, 37]. Failure to precisely measure the folate exposure in the periconceptional period is likely to yield imprecise results.
Current guidelines and recommendations for folic acid supplementation vary between 0.4 and 5 mg daily before and during the first 12 weeks or the entire pregnancy. Due to the high rates of unplanned pregnancies in individuals with epilepsy, some guidelines recommend at least 0.4 mg to all persons of childbearing potential using ASM. However, the challenge with recommending a fixed dose for all patients using ASM is that the individual need for folate differs depending on a variety of factors, including body mass index, genetic risk for low folate and genetic variations in folate-metabolizing enzymes, ethnicity, specific folic acid fortification practices, and adherence to supplementation recommendations [4, 11]. Medical factors such as the type and dose of ASMs, vitamin B12, B6, and B2 status, and conditions related to malabsorption could also influence folate concentrations. An individualized option is to titrate the folic acid dose according to concentrations of folate and possibly plasma homocysteine, the metabolic marker of folate deficiency. It is documented that a serum folate concentration of > 28–30 nmol/L, or red blood cell folate concentration above 906 nmol/L, reduces the risk of congenital malformations [11, 15]. This strategy should ensure a sufficient maternal concentration of folate regardless of country-wise folic acid fortification and individual risk factors for low folate concentrations [15], and at the same time should avoid excessive supplementation. The dose required for people using ASM to reach this concentration has not been investigated, and guidelines for persons with epilepsy have yet to include recommendations about folate measurements.
Randomized clinical trials comparing different folic acid doses in pregnant persons with epilepsy will require large study groups. As such studies are yet to be performed, future research should focus on establishing (a) the optimal folate concentration in this group in order to prevent adverse child outcomes, and (b) the dose needed to reach this concentration so as to maximize benefit and to avoid harm.
Practical conclusion
-
Folate is most likely important for the outcome in children of mothers on antiseizure medication (ASM). Folate status should be optimized before conception and throughout pregnancy.
-
The optimal dose of ASM is unknown, but recent guidelines recommend doses of 0.4–4 mg daily before pregnancy and at least during the first 12 gestational weeks. As the safety of excess folic acid is questioned, supplementation higher than needed should be avoided. In pregnant people without epilepsy, the optimal serum folate concentration is > 28–30 nmol/L or red blood cell concentration > 906 nmol/L. Folic acid dose titration according to the folate concentration is a possible strategy in pregnancy management of persons with ASM-treated epilepsy.
-
Folic acid supplements could mask vitamin B12 deficiency, which should also be measured before starting folic acid supplementation.
-
Future studies should focus on the efficacy and safety of individualizing folic acid dose according to concentration measurements.
References
Gerard EE, Meador KJ (2016) Managing epilepsy in women. Continuum (Minneap Minn) 22:204–226
Dwyer ER, Filion KB, MacFarlane AJ, Platt RW, Mehrabadi A (2022) Who should consume high-dose folic acid supplements before and during early pregnancy for the prevention of neural tube defects? BMJ 377:e67728
McNulty H, Ward M, Hoey L, Hughes CF, Pentieva K (2019) Addressing optimal folate and related B‑vitamin status through the lifecycle: health impacts and challenges. Proc Nutr Soc 78(3):449–462. https://doi.org/10.1017/S0029665119000661
Husebye ESN, Riedel B, Bjorke-Monsen AL et al (2021) Vitamin B status and association with antiseizure medication in pregnant women with epilepsy. Epilepsia 62(12):2968–2980. https://doi.org/10.1111/epi.17076
SIGN Diagnosis and management of epilepsy in adults: a national clinical guideline. https://www.sign.ac.uk/media/1079/sign143_2018.pdf. Accessed 03.01.
NICE Epilepsies in children, young people and adults. https://www.nice.org.uk/guidance/ng217/resources/epilepsies-in-children-young-people-and-adults-pdf-66143780239813. Accessed 03.01.
Sundhedsstyrelsen Referenceprogram for epilepsi. https://www.sst.dk/~/media/BE8541443FC8498BBF2B92746F9061A4.ashx. Accessed 03.01.
Swedish Medical Products Agency Läkemedel vid epilepsi—behandlings-rekommendation. https://www.lakemedelsverket.se/sv/behandling-och-forskrivning/behandlingsrekommendationer/sok-behandlingsrekommendationer/lakemedel-vid-epilepsi--behandlingsrekommendation. Accessed 03.01.
National centre for Epilepsy Planlegging av svangerskap og fødsel. https://epilepsibehandling.no/index.php?action=topic&item=JhraRspk. Accessed 03.01.
Tomson T, Battino D, Bromley R et al (2020) Global survey of guidelines for the management of epilepsy in pregnancy: a report from the international league against epilepsy task force on women and pregnancy. Epilepsia Open 5:366–370
Cordero AM, Crider KS, Rogers LM, Cannon MJ, Berry RJ (2015) Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health Organization guidelines. MMWR Morb Mortal Wkly Rep 64:421–423
Bjorke-Monsen AL, Renstrom R (2020) What is optimal folate status? Tidsskr Nor Laegeforen. https://doi.org/10.4045/tidsskr.19.0588
McGarel C, Pentieva K, Strain JJ, McNulty H (2015) Emerging roles for folate and related B‑vitamins in brain health across the lifecycle. Proc Nutr Soc 74:46–55
Bailey LB, Stover PJ, McNulty H et al (2015) Biomarkers of nutrition for development-folate review. J Nutr 145:1636S–1680S
Wilson RD, O’Connor DL (2022) Guideline no. 427: folic acid and multivitamin supplementation for prevention of folic acid-sensitive congenital anomalies. J Obstet Gynaecol Can 44:707–719.e1
Haggarty P UK introduces folic acid fortification of flour to prevent neural tube defects. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02134-6/fulltext. Accessed 22.09.
Brämswig S, Prinz-Langenohl R, Lamers Y et al (2009) Supplementation with a multivitamin containing 800 microg of folic acid shortens the time to reach the preventive red blood cell folate concentration in healthy women. Int J Vitam Nutr Res 79:61–70
Hursthouse NA, Gray AR, Miller JC, Rose MC, Houghton LA (2011) Folate status of reproductive age women and neural tube defect risk: the effect of long-term folic acid supplementation at doses of 140 µg and 400 µg per day. Nutrients 3:49–62
Reynolds EH (2020) Antiepileptic drugs, folate and one carbon metabolism revisited. Epilepsy Behav 112:107336
Walker DI, Perry-Walker K, Finnell RH et al (2019) Metabolome-wide association study of anti-epileptic drug treatment during pregnancy. Toxicol Appl Pharmacol 363:122–130
Reynolds EH, Green R (2020) Valproate and folate: congenital and developmental risks. Epilepsy Behav 108:107068
Muhsen M, Youngs J, Riu A et al (2021) Folic acid supplementation rescues valproic acid-induced developmental neurotoxicity and behavioral alterations in zebrafish embryos. Epilepsia 62(7):1689–1700. https://doi.org/10.1111/epi.16915
Abdulrazzaq YM, Shafiullah M, Kochyil J, Padmanabhan R, Bastaki SMA (2018) Ameliorative effects of supplemental folinic acid on lamotrigine-induced fetal malformations in the mouse. Mol Cell Biochem 446(1):185–197. https://doi.org/10.1007/s11010-018-3285-0
Berman E, Kohn E, Berkovitch M, Kovo M, Eyal S (2022) Lacosamide effects on placental carriers of essential compounds in comparison with valproate: studies in perfused human placentas. Epilepsia 63:2949–2957
Kardoost M, Hajizadeh-Saffar E, Ghorbanian MT et al (2019) Genotoxicity assessment of antiepileptic drugs (AEDs) in human embryonic stem cells. Epilepsy Res 158:106232
Li Y, Meador KJ (2022) Epilepsy and pregnancy. Continuum (Minneap Minn) 28:34–54
Alvestad S, Husebye ESN, Christensen J et al (2022) Folic acid and risk of preterm birth, preeclampsia, and fetal growth restriction among women with epilepsy: a prospective cohort study. Neurology 99:e605–e615
Morrow JI, Hunt SJ, Russell AJ et al (2009) Folic acid use and major congenital malformations in offspring of women with epilepsy: a prospective study from the UK epilepsy and pregnancy register. J Neurol Neurosurg Psychiatry 80:506–511
Tomson T, Battino D, Bonizzoni E et al (2018) Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol 17:530–538
Campbell E, Kennedy F, Russell A et al (2014) Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and ireland epilepsy and pregnancy registers. J Neurol Neurosurg Psychiatry 85:1029–1034
Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA (2000) Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med 343:1608–1614
Kjaer D, Horvath-Puho E, Christensen J et al (2008) Antiepileptic drug use, folic acid supplementation, and congenital abnormalities: a population-based case-control study. BJOG 115:98–103
Pittschieler S, Brezinka C, Jahn B et al (2008) Spontaneous abortion and the prophylactic effect of folic acid supplementation in epileptic women undergoing antiepileptic therapy. J Neurol 255:1926–1931
Tomson T, Battino D, Bonizzoni E et al (2015) Antiepileptic drugs and intrauterine death: a prospective observational study from EURAP. Neurology 85:580–588
Dansky LV, Andermann E, Rosenblatt D, Sherwin AL, Andermann F (1987) Anticonvulsants, folate levels, and pregnancy outcome: a prospective study. Ann Neurol 21:176–182
Dean J, Robertson Z, Reid V et al (2007) Fetal anticonvulsant syndromes and polymorphisms in MTHFR, MTR, and MTRR. Am J Med Genet A 143A:2303–2311
Meador KJ, Baker GA, Browning N et al (2013) Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol 12:244–252
Meador KJ, Pennell PB, May RC et al (2020) Effects of periconceptional folate on cognition in children of women with epilepsy: NEAD study. Neurology 94:e729–e740
Sadat-Hossieny Z, Robalino CP, Pennell PB et al (2021) Folate fortification of food: insufficient for women with epilepsy. Epilepsy Behav 117:107688
Bjork M, Riedel B, Spigset O et al (2018) Association of folic acid supplementation during pregnancy with the risk of autistic traits in children exposed to antiepileptic drugs in utero. JAMA Neurol 75:160–168
Husebye ESN, Gilhus NE, Riedel B, Spigset O, Daltveit AK, Bjork MH (2018) Verbal abilities in children of mothers with epilepsy: association to maternal folate status. Neurology 91:e811–e821
Husebye ESN, Gilhus NE, Spigset O, Daltveit AK, Bjork MH (2020) Language impairment in children aged 5 and 8 years after antiepileptic drug exposure in utero—the Norwegian mother and child cohort study. Eur J Neurol 27:667–675
Wood AG, Nadebaum C, Anderson V et al (2015) Prospective assessment of autism traits in children exposed to antiepileptic drugs during pregnancy. Epilepsia 56:1047–1055
Baker GA, Bromley RL, Briggs M et al (2015) IQ at 6 years after in utero exposure to antiepileptic drugs: a controlled cohort study. Neurology 84:382–390
Huber-Mollema Y, van Iterson L, Oort FJ, Lindhout D, Rodenburg R (2020) Neurocognition after prenatal levetiracetam, lamotrigine, carbamazepine or valproate exposure. J Neurol 267:1724–1736
Kasradze S, Gogatishvili N, Lomidze G et al (2017) Cognitive functions in children exposed to antiepileptic drugs in utero—study in Georgia. Epilepsy Behav 66:105–112
Field MS, Stover PJ (2018) Safety of folic acid. Ann N Y Acad Sci 1414:59–71
Wien TN, Pike E, Wisloff T, Staff A, Smeland S, Klemp M (2012) Cancer risk with folic acid supplements: a systematic review and meta-analysis. BMJ Open 2:e653
Cao X, Xu J, Lin YL et al (2023) Excess folic acid intake increases DNA de novo point mutations. Cell Discov 9:22
Vollset SE, Clarke R, Lewington S et al (2013) Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet 381:1029–1036
Mortensen JH, Oyen N, Fomina T et al (2015) Supplemental folic acid in pregnancy and maternal cancer risk. Cancer Epidemiol 39:805–811
EFSA Scientific opinion on dietary reference values for folate. https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2014.3893. Accessed 15 December 2022
Vegrim HM, Dreier JW, Alvestad S et al (2022) Cancer risk in children of mothers with epilepsy and high-dose folic acid use during pregnancy. JAMA Neurol 79:1–10
Murray LK, Smith MJ, Jadavji NM (2018) Maternal oversupplementation with folic acid and its impact on neurodevelopment of offspring. Nutr Rev 76:708–721
Maruvada P, Stover PJ, Mason JB et al (2020) Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am J Clin Nutr 112:1390–1403
Raghavan R, Riley AW, Volk H et al (2018) Maternal multivitamin intake, plasma folate and vitamin B(12) levels and autism spectrum disorder risk in offspring. Paediatr Perinat Epidemiol 32:100–111
Raghavan R, Selhub J, Paul L et al (2020) A prospective birth cohort study on cord blood folate subtypes and risk of autism spectrum disorder. Am J Clin Nutr 112(5):1304–1317. https://doi.org/10.1093/ajcn/nqaa208
Harlan De Crescenzo A, Panoutsopoulos AA, Tat L et al (2021) Deficient or excess folic acid supply during pregnancy alter cortical neurodevelopment in mouse offspring. Cereb Cortex 31:635–649
Valera-Gran D, Garcia de la Hera M, Navarrete-Munoz EM et al (2014) Folic acid supplements during pregnancy and child psychomotor development after the first year of life. JAMA Pediatr 168:e142611
Husebye ESN, Wendel AWK, Gilhus NE, Riedel B, Bjørk MH (2022) Plasma unmetabolized folic acid in pregnancy and risk of autistic traits and language impairment in antiseizure medication-exposed children of women with epilepsy. Am J Clin Nutr 115(5):1432–1440. https://doi.org/10.1093/ajcn/nqab436
Tomson T, Battino D, Bromley R et al (2019) Management of epilepsy in pregnancy: a report from the international league against epilepsy task force on women and pregnancy. Epileptic Disord 21:497–517
American College of Obstetricians and Gynecologists (2017) Practice bulletin no. 187 summary: neural tube defects. Obstet Gynecol 130(6):e279–e290
Harden CL, Pennell PB, Koppel BS et al (2009) Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the quality standards subcommittee and therapeutics and technology assessment subcommittee of the American academy of neurology and American epilepsy society. Neurology 73:142–149
Pennell P Management of epilepsy during preconception, pregnancy, and the postpartum period. https://www.uptodate.com/contents/management-of-epilepsy-during-preconception-pregnancy-and-the-postpartum-period#H2835490360. Accessed 24.02.
Epilepsiat (aikuiset) Current care guidelines. https://www.kaypahoito.fi/hoi50072. Accessed 28.03.
The National University Hospital of Iceland Flogaveiki og meðganga. https://www.landspitali.is/library/Sameiginlegar-skrar/Gagnasafn/Sjuklingar-og-adstandendur/Sjuklingafraedsla---Upplysingarit/Kvennadeildir/flogaveiki_og_medganga_2019.pdf. Accessed 28.03.
Bjork M, Nordeng H, Alvestad S et al (2018) Preconception guidance, teratogenecity, pregnancy, delivery In. In: Sveberg L (ed) Treatment guideline for women with epilepsy, 3rd edn. The Norwegian Medical Assosiation, Oslo
Johnson EL, Burke AE, Wang A, Pennell PB (2018) Unintended pregnancy, prenatal care, newborn outcomes, and breastfeeding in women with epilepsy. Neurology 91:e1031–e1039
MRC Vitamin Study Research Group (1991) Prevention of neural tube defects: results of the medical research council vitamin study. Lancet 338:131–137
Vegrim HM, Tomson T, Bjork MH (2023) Benefits and risks of periconceptional folic acid supplementation-reply. JAMA Neurol 80(4):421–422. https://doi.org/10.1001/jamaneurol.2023.0092
Funding
Open access funding provided by University of Bergen (incl Haukeland University Hospital)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.-H. Bjørk, H. Vegrim, S. Alvestad, A.-L. Bjørke-Monsen, B. Riedel, N. E. Gilhus and E. S. N. Husebye declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information

Scan QR code & read article online
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bjørk, MH., Vegrim, H., Alvestad, S. et al. Pregnancy, folic acid, and antiseizure medication. Clin Epileptol 36, 203–211 (2023). https://doi.org/10.1007/s10309-023-00602-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10309-023-00602-3