Abstract
Purpose
This systematic review aimed to evaluate the effect of transcutaneous auricular vagus nerve stimulation on heart rate variability and baroreflex sensitivity in healthy populations.
Method
PubMed, Scopus, the Cochrane Library, Embase, and Web of Science were systematically searched for controlled trials that examined the effects of transcutaneous auricular vagus nerve stimulation on heart rate variability parameters and baroreflex sensitivity in apparently healthy individuals. Two independent researchers screened the search results, extracted the data, and evaluated the quality of the included studies.
Results
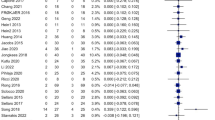
From 2458 screened studies, 21 were included. Compared with baseline measures or the comparison group, significant changes in the standard deviation of NN intervals, the root mean square of successive RR intervals, the proportion of consecutive RR intervals that differ by more than 50 ms, high-frequency power, low-frequency to high-frequency ratio, and low-frequency power were found in 86%, 75%, 69%, 47%, 36%, and 25% of the studies evaluating the effects of transcutaneous auricular vagus nerve stimulation on these indices, respectively. Baroreflex sensitivity was evaluated in six studies, of which a significant change was detected in only one. Some studies have shown that the worse the basic autonomic function, the better the response to transcutaneous auricular vagus nerve stimulation.
Conclusion
The results were mixed, which may be mainly attributable to the heterogeneity of the study designs and stimulation delivery dosages. Thus, future studies with comparable designs are required to determine the optimal stimulation parameters and clarify the significance of autonomic indices as a reliable marker of neuromodulation responsiveness.
Graphical abstract










Similar content being viewed by others
References
Weir HK, Anderson RN, Coleman King SM, Soman A, Thompson TD, Hong Y et al (2016) Heart disease and cancer deaths - trends and projections in the United States, 1969–2020. Prev Chronic Dis 13:E157. https://doi.org/10.5888/pcd13.160211
Xu J, Murphy SL, Kochanek KD, Arias E (2015) Mortality in the United States. NCHS Data Brief 2016(267):1–8
Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB et al (2003) Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. Jama 290(7):891–7. https://doi.org/10.1001/jama.290.7.891
Kaniusas E, Kampusch S, Tittgemeyer M, Panetsos F, Gines RF, Papa M et al (2019) Current directions in the auricular vagus nerve stimulation i - a physiological perspective. Front Neurosci 13:854. https://doi.org/10.3389/fnins.2019.00854
Beekwilder JP, Beems T (2010) Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol. 27(2):130–8. https://doi.org/10.1097/WNP.0b013e3181d64d8a
Pellissier S, Dantzer C, Canini F, Mathieu N, Bonaz B (2010) Psychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndrome. Psychoneuroendocrinology. 35(5):653–62. https://doi.org/10.1016/j.psyneuen.2009.10.004
Curtis BM, Oeefe JH Jr (2002) Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc 77:45–54
Antonino D, Teixeira AL, Maia-Lopes PM, Souza MC, Sabino-Carvalho JL, Murray AR et al (2017) Non-invasive vagus nerve stimulation acutely improves spontaneous cardiac baroreflex sensitivity in healthy young men: a randomized placebo-controlled trial. Brain Stimul 10(5):875–881. https://doi.org/10.1016/j.brs.2017.05.006
Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J (2014) Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul 7(6):871–877. https://doi.org/10.1016/j.brs.2014.07.031
Chen M, Wang S, Li X, Yu L, Yang H, Liu Q et al (2020) Non-invasive autonomic neuromodulation is opening new landscapes for cardiovascular diseases. Front Physiol 11:550578. https://doi.org/10.3389/fphys.2020.550578
Naftchi NE (1990) Mechanism of autonomic dysreflexia. Contributions of catecholamine and peptide neurotransmitters. Ann N Y Acad Sci. 579:133–48. https://doi.org/10.1111/j.1749-6632.1990.tb48356.x
Chen M, Zhou X, Yu L, Liu Q, Sheng X, Wang Z et al (2016) Low-level vagus nerve stimulation attenuates myocardial ischemic reperfusion injury by antioxidative stress and antiapoptosis reactions in canines. J Cardiovasc Electrophysiol 27(2):224–31. https://doi.org/10.1111/jce.12850
Sheng X, Chen M, Huang B, Liu J, Zhou L, Bao M et al (2016) Cardioprotective effects of low-level carotid baroreceptor stimulation against myocardial ischemia-reperfusion injury in canine model. J Interv Card Electrophysiol 45(2):131–40. https://doi.org/10.1007/s10840-015-0094-1
Lopshire JC, Zipes DP (2014) Spinal cord stimulation for heart failure: preclinical studies to determine optimal stimulation parameters for clinical efficacy. J Cardiovasc Transl Res 7(3):321–9. https://doi.org/10.1007/s12265-014-9547-7
Zipes DP (2017) Ablation of atrial gangionated plexi to treat symptomatic sinus bradycardia. JACC Clin Electrophysiol 3(9):960–961. https://doi.org/10.1016/j.jacep.2017.02.010
Böhm M, Linz D, Urban D, Mahfoud F, Ukena C (2013) Renal sympathetic denervation: applications in hypertension and beyond. Nat Rev Cardiol 10(8):465–76. https://doi.org/10.1038/nrcardio.2013.89
Cha YM, Li X, Yang M, Han J, Wu G, Kapa SC et al (2019) Stellate ganglion block and cardiac sympathetic denervation in patients with inappropriate sinus tachycardia. J Cardiovasc Electrophysiol 30(12):2920–2928. https://doi.org/10.1111/jce.14233
Soltani D, Samimi S, Vasheghani-Farahani A, Shariatpanahi SP, Abdolmaleki P, Madjid Ansari A (2023) Electromagnetic field therapy in cardiovascular diseases: a review of patents, clinically effective devices, and mechanism of therapeutic effects. Trends Cardiovasc Med 33(2):72–78. https://doi.org/10.1016/j.tcm.2021.10.006
Borges U, Pfannenstiel M, Tsukahara J, Laborde S, Klatt S, Raab M (2021) Transcutaneous vagus nerve stimulation via tragus or cymba conchae: are its psychophysiological effects dependent on the stimulation area? Int J Psychophysiol 161:64–75. https://doi.org/10.1016/j.ijpsycho.2021.01.003
De Couck M, Cserjesi R, Caers R, Zijlstra WP, Widjaja D, Wolf N et al (2017) Effects of short and prolonged transcutaneous vagus nerve stimulation on heart rate variability in healthy subjects. Auton Neurosci 203:88–96. https://doi.org/10.1016/j.autneu.2016.11.003
Borges U, Laborde S, Raab M (2019) Influence of transcutaneous vagus nerve stimulation on cardiac vagal activity: Not different from sham stimulation and no effect of stimulation intensity. PLoS One 14(10):e0223848. https://doi.org/10.1371/journal.pone.0223848
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372:n71. https://doi.org/10.1136/bmj.n71
Higgins JP, Savović J, Page MJ, Sterne JA (2021) Revised Cochrane risk of bias tool for randomized trials (RoB 2) additional considerations for crossover trials. RoB2 Development Group [Internet]
Sterne J A, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. bmj 366
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al (2019) Cochrane handbook for systematic reviews of interventions. John Wiley & Sons
Gauthey A, Morra S, van de Borne P, Deriaz D, Maes N, le Polain JB, de Waroux, (2020) Sympathetic effect of auricular transcutaneous vagus nerve stimulation on healthy subjects: a crossover controlled clinical trial comparing vagally mediated and active control stimulation using microneurography. Front Physiol. https://doi.org/10.3389/fphys.2020.599896
Sinkovec M, Trobec R, Meglic B (2021) Cardiovascular responses to low-level transcutaneous vagus nerve stimulation. Auton Neurosci Basic Clin. https://doi.org/10.1016/j.autneu.2021.102851
Vosseler A, Zhao D, Fritsche L, Lehmann R, Kantartzis K, Small DM et al (2020) No modulation of postprandial metabolism by transcutaneous auricular vagus nerve stimulation: a cross-over study in 15 healthy men. Sci Rep 10(1):20466. https://doi.org/10.1038/s41598-020-77430-2
De Couck M, Cserjesi R, Caers R, Zijlstra WP, Widjaja D, Wolf N et al (2017) Effects of short and prolonged transcutaneous vagus nerve stimulation on heart rate variability in healthy subjects. Auton Neurosci Basic Clin 203:88–96. https://doi.org/10.1016/j.autneu.2016.11.003
De Smet S, Ottaviani C, Verkuil B, Kappen M, Baeken C, Vanderhasselt MA (2023) Effects of non-invasive vagus nerve stimulation on cognitive and autonomic correlates of perseverative cognition. Psychophysiology. https://doi.org/10.1111/psyp.14250
Forte G, Favieri F, Leemhuis E, De Martino ML, Giannini AM, De Gennaro L et al (2022) Ear your heart: transcutaneous auricular vagus nerve stimulation on heart rate variability in healthy young participants. PeerJ 10:e14447. https://doi.org/10.7717/peerj.14447
Geng D, Liu X, Wang Y, Wang J (2022) The effect of transcutaneous auricular vagus nerve stimulation on HRV in healthy young people. PLoS One 17(2):e0263833. https://doi.org/10.1371/journal.pone.0263833
Keute M, Machetanz K, Berelidze L, Guggenberger R, Gharabaghi A (2021) Neuro-cardiac coupling predicts transcutaneous auricular vagus nerve stimulation effects. Brain Stimul 14(2):209–216. https://doi.org/10.1016/j.brs.2021.01.001
Kozorosky EM, Lee CH, Lee JG, Nunez Martinez V, Padayachee LE, Stauss HM (2022) Transcutaneous auricular vagus nerve stimulation augments postprandial inhibition of ghrelin. Physiol Rep. https://doi.org/10.14814/phy2.15253
Sclocco R, Garcia RG, Kettner NW, Fisher HP, Isenburg K, Makarovsky M et al (2020) Stimulus frequency modulates brainstem response to respiratory-gated transcutaneous auricular vagus nerve stimulation. Brain Stimul 13(4):970–978. https://doi.org/10.1016/j.brs.2020.03.011
Shen LL, Sun JB, Yang XJ, Deng H, Qin W, Du MY et al (2022) Reassessment of the effect of transcutaneous auricular vagus nerve stimulation using a novel burst paradigm on cardiac autonomic function in healthy young adults. Neuromodulation 25(3):433–442. https://doi.org/10.1111/ner.13521
Villani V, Tsakiris M, Azevedo R (2019) Transcutaneous vagus nerve stimulation improves interoceptive accuracy. Neuropsychologia 134:107201
Zhu Y, Xu F, Sun C, Xu W, Li M, Gong Y et al (2022) Noninvasive transcutaneous auricular vagal nerve stimulation improves gastric slow waves impaired by cold stress in healthy subjects. Neuromodulation. https://doi.org/10.1016/j.neurom.2022.03.010
Dalgleish AS, Kania AM, Stauss HM, Jelen AZ (2021) Occipitoatlantal decompression and noninvasive vagus nerve stimulation slow conduction velocity through the atrioventricular node in healthy participants. J Osteopath Med 121(4):349–359. https://doi.org/10.1515/jom-2020-0213
Gancheva S, Bierwagen A, Markgraf DF, Bönhof GJ, Murphy KG, Hatziagelaki E et al (2018) Constant hepatic ATP concentrations during prolonged fasting and absence of effects of Cerbomed Nemos® on parasympathetic tone and hepatic energy metabolism. Mol Metab 7:71–79. https://doi.org/10.1016/j.molmet.2017.10.002
Kania AM, Weiler KN, Kurian AP, Opena ML, Orellana JN, Stauss HM (2021) Activation of the cholinergic antiinflammatory reflex by occipitoatlantal decompression and transcutaneous auricular vagus nerve stimulation. J Osteopath Med 121(4):401–415. https://doi.org/10.1515/jom-2020-0071
Bretherton B, Atkinson L, Murray A, Clancy J, Deuchars S, Deuchars J (2019) Effects of transcutaneous vagus nerve stimulation in individuals aged 55 years or above: potential benefits of daily stimulation. Aging (Albany NY) 11(14):4836–4857
Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ (1987) Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59(4):256–262
Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M et al (1998) Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation 98(15):1510–1516
Johansson M, Gao SA, Friberg P, Annerstedt M, Carlström J, Ivarsson T et al (2007) Baroreflex effectiveness index and baroreflex sensitivity predict all-cause mortality and sudden death in hypertensive patients with chronic renal failure. J Hypertens 25(1):163–168
La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ (1998) Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. The Lancet 351(9101):478–484
Jouven X, Empana J-P, Schwartz PJ, Desnos M, Courbon D, Ducimetière P (2005) Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 352(19):1951–1958
Kiviniemi AM, Tulppo MP, Hautala AJ, Perkiömäki JS, Ylitalo A, Kesäniemi YA et al (2014) Prognostic significance of impaired baroreflex sensitivity assessed from Phase IV of the Valsalva maneuver in a population-based sample of middle-aged subjects. Am J Cardiol 114(4):571–576
Mischel NA, Mueller PJ (2011) (In)activity-dependent alterations in resting and reflex control of splanchnic sympathetic nerve activity. J Appl Physiol 111(6):1854–1862
Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J (2009) The sympathetic nervous system in heart failure: physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54(19):1747–1762
Shaffer FM, Ginsberg JP (2017) An overview of heart rate variability metrics and norms. Front Public Health 5:258
Task Force of the European Society of Cardiology the North A Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93(5):1043–1065
Shaffer F, McCraty R, Zerr CL (2014) A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol 5:1040
Mertens A, Carrette S, Klooster D, Lescrauwaet E, Delbeke J, Wadman WJ et al (2022) Investigating the effect of transcutaneous auricular vagus nerve stimulation on cortical excitability in healthy males. Neuromodulation Technol Neural Interface 5(3):395–406
Machetanz K, Berelidze L, Guggenberger R, Gharabaghi A (2021) Transcutaneous auricular vagus nerve stimulation and heart rate variability: analysis of parameters and targets. Auton Neurosci 236:102894
Umetani K, Singer DH, McCraty R, Atkinson M (1998) Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol 31(3):593–601
Messina G, Vicidomini C, Viggiano A, Tafuri D, Cozza V, Cibelli G et al (2012) Enhanced parasympathetic activity of sportive women is paradoxically associated to enhanced resting energy expenditure. Auton Neurosci 169(2):102–106
De Couck M, Mravec B, Gidron Y (2012) You may need the vagus nerve to understand pathophysiology and to treat diseases. Clin Sci 122(7):323–328
Entschladen F, Drell TL, Lang K, Joseph J, Zaenker KS (2004) Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncol 5(4):254–258
Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE (2008) The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology 33(10):1305–1312
Tsutsumi T, Ide T, Yamato M, Kudou W, Andou M, Hirooka Y et al (2008) Modulation of the myocardial redox state by vagal nerve stimulation after experimental myocardial infarction. Cardiovasc Res 77(4):713–721
Vlcek M, Radikova Z, Penesova A, Kvetnansky R, Imrich R (2008) Heart rate variability and catecholamines during hypoglycemia and orthostasis. Auton Neurosci 143(1–2):53–57
Fallgatter AJ, Neuhauser B, Herrmann MJ, Ehlis AC, Wagener A, Scheuerpflug P et al (2003) Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm (Vienna) 110(12):1437–43. https://doi.org/10.1007/s00702-003-0087-6
Uradu A, Wan J, Doytchinova A, Wright KC, Lin AYT, Chen LS et al (2017) Skin sympathetic nerve activity precedes the onset and termination of paroxysmal atrial tachycardia and fibrillation. Heart Rhythm 14(7):964–971. https://doi.org/10.1016/j.hrthm.2017.03.030
Shen LL, Sun JB, Yang XJ, Deng H, Qin W, Du MY et al (2021) Reassessment of the effect of transcutaneous auricular vagus nerve stimulation using a novel burst paradigm on cardiac autonomic function in healthy young adults. Neuromodulation 25(3):433–442. https://doi.org/10.1111/ner.13521
Barantke M, Krauss T, Ortak J, Lieb W, Reppel M, Burgdorf C et al (2008) Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J Cardiovasc Electrophysiol 19(12):1296–1303
Abhishekh HA, Nisarga P, Kisan R, Meghana A, Chandran S, Raju T et al (2013) Influence of age and gender on autonomic regulation of heart. J Clin Monit Comput 27:259–264
Almeida-Santos MA, Barreto-Filho JA, Oliveira JLM, Reis FP, da Cunha Oliveira CC, Sousa ACS (2016) Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch Gerontol Geriatr 63:1–8
Koenig J, Thayer JF (2016) Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci Biobehav Rev 64:288–310
Krames E, Peckham PH, Rezai AR (2018) Neuromodulation: comprehensive textbook of principles, technologies, and therapies. Academic Press
Polak T, Markulin F, Ehlis AC, Langer JB, Ringel TM, Fallgatter AJ (2009) Far field potentials from brain stem after transcutaneous vagus nerve stimulation: optimization of stimulation and recording parameters. J Neural Transm 116:1237–1242
Kaniusas E, Kampusch S, Tittgemeyer M, Panetsos F, Gines RF, Papa M, et al. (2019) Current directions in the auricular vagus nerve stimulation I–a physiological perspective. Frontiers in neuroscience 854
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The manuscript does not contain clinical studies or patient data.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soltani, D., Azizi, B., Sima, S. et al. A systematic review of the effects of transcutaneous auricular vagus nerve stimulation on baroreflex sensitivity and heart rate variability in healthy subjects. Clin Auton Res 33, 165–189 (2023). https://doi.org/10.1007/s10286-023-00938-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-023-00938-w