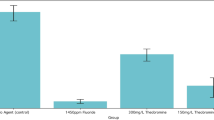
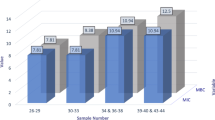
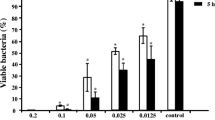
Abstract
Dental caries is a type of oral microbiome dysbiosis and biofilm infection that affects oral and systemic conditions. For healthy life expectancy, natural bacteriostatic products are ideal for daily and lifetime use as anti-oral infection agents. This study aimed to evaluate the inhibitory effects of abietic acid, a diterpene derived from pine rosin, on the in vitro growth of cariogenic bacterial species, Streptococcus mutans. The effective minimum inhibitory concentration of abietic acid was determined through observation of S. mutans growth, acidification, and biofilm formation. The inhibitory effects of abietic acid on the bacterial membrane were investigated through the use of in situ viability analysis and scanning electron microscopic analysis. Cytotoxicity of abietic acid was also examined in the context of several human cell lines using tetrazolium reduction assay. Abietic acid was found to inhibit key bacterial growth hallmarks such as colony forming ability, adenosine triphosphate activity (both planktonic and biofilm), acid production, and biofilm formation. Abietic acid was identified as bacteriostatic, and this compound caused minimal damage to the bacterial membrane. This action was different from that of povidone-iodine or cetylpyridinium chloride. Additionally, abietic acid was significantly less cytotoxic compared to povidone-iodine, and it exerted lower toxicity towards epithelial cells and fibroblasts compared to that against monocytic cells. These data suggest that abietic acid may prove useful as an antibacterial and antibiofilm agent for controlling S. mutans infection.





Similar content being viewed by others
References
Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32.
Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontology. 2000;2006(42):80–7.
Cisar JO, Kolenbrander PE, McIntire FC. Specificity of coaggregation reactions between human oral Streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979;24:742–52.
Simón-Soro A, Mira A. Solving the etiology of dental caries. Trends Microbiol. 2015;23(2):76–82.
Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45(1):69–86.
Metwalli KH, Khan SA, Krom BP, Jabra-Rizk MA. Streptococcus mutans, Candida albicans, and the human mouth: a sticky situation. PLoS Pathog. 2013;9(10):e1003616.
Nakano K, Hokamura K, Taniguchi N, Wada K, Kudo C, Nomura R, Kojima A, Naka S, Muranaka Y, Thura M, Nakajima A, Masuda K, Nakagawa I, Speziale P, Shimada N, Amano A, Kamisaki Y, Tanaka T, Umemura K, Ooshima T. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat Commun. 2011;2:485.
Kojima A, Nakano K, Wada K, Takahashi H, Katayama K, Yoneda M, Higurashi T, Nomura R, Hokamura K, Muranaka Y, Matsuhashi N, Umemura K, Kamisaki Y, Nakajima A, Ooshima T. Infection of specific strains of Streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci Rep. 2012;2:332.
Watanabe I, Kuriyama N, Miyatani F, Nomura R, Naka S, Nakano K, Ihara M, Iwai K, Matsui D, Ozaki E, Koyama T, Nishigaki M, Yamamoto T, Tamura A, Mizuno T, Akazawa K, Takada A, Takeda K, Yamada K, Nakagawa M, Tanaka T, Kanamura N, Friedland RP, Watanabe Y. Oral Cnm-positive Streptococcus Mutans expressing collagen binding activity is a risk factor for cerebral microbleeds and cognitive impairment. Sci Rep. 2016;9(6):38561.
Fung-Tomc J. Correlation of in vitro and in vivo resistance development to antimicrobial agents. Antimicrob Newslett. 1990;7:17–24.
Pallasch TJ, Slots J. Antibiotic prophylaxis and the medically compromised host. Periodontology. 2000;1996(10):107–38.
Scully C, El-Kabir M, Samaranayake LP. Candida and oral candidosis: a review. Crit Rev Oral Biol Med. 1994;5:125–57.
Tacconelli E, De Angelis G, Cataldo MA, Pozzi E, Cauda R. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. J Antimicrob Chemother. 2008;61:26–38.
Brecx M, Netuschil L, Hoffmann T. How to select the right mouthrinses in periodontal prevention and therapy. Part II. Clinical use and recommendations. Int J Dent Hyg. 2003;1(4):188–94.
Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA. Povidone iodine in wound healing: a review of current concepts and practices. Int J Surg. 2017;44:260–8.
Labeau SO, Van de Vyver K, Brusselaers N, Vogelaers D, Blot SI. Prevention of ventilator-associated pneumonia with oral antiseptics: a systematic review and meta-analysis. Lancet Infect Dis. 2011;11(11):845–54.
Addy M, Wright R. Comparison of the in vivo and in vitro antibacterial properties of providone iodine and chlorhexidine gluconate mouthrinses. J Clin Periodontol. 1978;5(3):198–205.
Jones CG. Chlorhexidine: is it still the gold standard? Periodontology 2000. 1997;15:55–62.
Krautheim AB, Jermann TH, Bircher AJ. Chlorhexidine anaphylaxis: case report and review of the literature. Contact Dermat. 2004;50(3):113–6.
Aremu OS, Gopaul K, Kadam P, Singh M, Mocktar C, Singh P, Koorbanally NA. Synthesis, characterization, anticancer and antibacterial activity of some novel pyrano [2,3-d] pyrimidinone carbonitrile derivatives. Anticancer Agents Med Chem. 2017;17:719–25.
Chung PY, Toh YS. Anti-biofilm agents: recent breakthrough against multi-drug resistant Staphylococcus aureus. Pathog Dis. 2014;70:231–9.
Perumal S, Mahmud R. Chemical analysis, inhibition of biofilm formation and biofilm eradication potential of Euphorbia hirta L. against clinical isolates and standard strains. BMC Complement Altern Med. 2013;13:346.
Wu CY, Su TY, Wang MY, Yang SF, Mar K, Hung SL. Inhibitory effects of tea catechin epigallocatechin-3-gallate against biofilms formed from Streptococcus mutans and a probiotic lactobacillus strain. Arch Oral Biol. 2018;26:69–77.
Keeling CI, Bohlmann J. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defense of confers against insects and pathogens. New Phytol. 2006;170:657–75.
Kitaoka N, Lu X, Yang B, Peters RJ. The application of synthetic biology to elucidation of Plant mono-, sesqui-, and diterpenoid metabolism. Mol Plant. 2015;8:6–16.
Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol®). J Biol Chem. 1997;272:2534–41.
Galeotti N, Di Cesare Mannelli L, Mazzanti G, Bartolini A, Ghelardini C. Menthol: a natural analgesic compound. Neurosci Lett. 2002;322(3):145–8.
Horiuchi K, Shiota S, Hatano T, Yoshida T, Kuroda T, Tsuchiya T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol Pharm Bull. 2007;30:1147–9.
Raut JS, Shinde RB, Chauhan NM, Karuppayil SM. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling. 2013;29:87–96.
Zeiss HH. The chemistry of the resin acids. Chem Rev. 1948;42:163–87.
Fernández MA, Tornos MP, García MD, de Heras B, Villar AM, Sáenz MT. Anti-inflammatory activity of abietic acid, a diterpene isolated from Pimenta recemosa var. grissea. J Pharm Pharmacol. 2001;53:867–72.
Kim NH, Son Y, Jeong SO, Hur JM, Bang HS, Lee KN, Kim EC, Chung HT, Pae HO. Tetrahydroabietic acid, a reduced abietic acid, inhibits the production of inflammatory mediators in RAW264.7 macrophages activated with lipopolysaccharide. J Clin Biochem Nutr. 2010;46:119–25.
Ukiya M, Kawaguchi T, Ishii K, Ogihara E, Tachi Y, Kurita M, Ezaki Y, Fukatsu M, Kushi Y, Akihisa T. Cytotoxic activities of amino acid-conjugate derivatives of abietane-type diterpenoids against human cancer cell lines. Chem Biodivers. 2013;10:1260–8.
Yoshida N, Takada T, Yamamura Y, Adachi I, Suzuki H, Kawakami J. Inhibitory effects of terpenoids on multidrug resistance-associated protein 2- and breast cancer resistance protein-mediated transport. Drug Metab Dispos. 2008;36:1206–11.
Ohtsu H, Tanaka R, In Y, Matsunaga S, Tokuda H, Nishino H. Abietane diterpenoids from the cones of Larix kaempferi and their inhibitory effects on Epstein-Barr virus activation. Planta Med. 2001;67:55–60.
Ganewatta MS, Miller KP, Singleton P, Mehrpouya-Bahrami P, Chen YP, Yan Y, Nagarkatti M, Nagarkatti P, Decho AW, Tang C. Antibacterial and biofilm-disrupting coatings from resin acid-derived materials. Biomacromol. 2015;16:3336–44.
Naruishi K, Takashiba S, Nishimura F, Chou HH, Arai H, Yamada H, Murayama Y. Impairment of gingival fibroblast adherence by IL-6/sIL-6R. J Dent Res. 2001;80:1421–4.
Matsumi Y, Fujita K, Takashima Y, Yanagida K, Morikawa Y, Matsumoto-Nakano M. Contribution of glucan-binding protein A to firm and stable biofilm formation by Streptococcus mutans. Mol Oral Microbiol. 2015;30:217–26.
Song JH, Kim SK, Chang KW, Han SK, Yi HK, Jeon JG. In vitro inhibitory effects of Polygonum cuspidatum on bacterial viability and virulence factors of Streptococcus mutans and Streptococcus sobrinus. Arch Oral Biol. 2006;51(12):1131–40 (Epub).
Almagor J, Temkin E, Benenson I, Fallach N, Carmeli Y, DRIVE-AB consortium. The impact of antibiotic use on transmission of resistant bacteria in hospitals: Insights from an agent-based model. PLoS One. 2018;13:e0197111.
World Health Organization (WHO). Antimicrobial resistance: global report on surveillance. Geneva: WHO; 2014.
Krzyściak W, Jurczak A, Kościelniak D, Bystrowska B, Skalniak A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis. 2014;33:499–515.
Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44(2):331–84.
He J, Li Y, Cao Y, Xue J, Zhou X. The oral microbiome diversity and its relation to human disease. Folia Microbiol. 2015;60:69–80.
Arimatsu K, Yamada H, Miyazawa H, Minagawa T, Nakajima M, Ryder MI, Gotoh K, Motooka D, Nakamura S, Iida T, Yamazaki K. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep. 2014;6:4828.
Kostic AG, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, Baselga J, Liu C, Shivdasani RA, Ogino S, Meyarson M. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–8.
Acknowledgements
The authors would like to thank Prof. Teruo Kuroda, Department of Microbiology, School of Pharmaceutical sciences, Hiroshima University, for his support from the beginning of this research. We would like to express our gratitude to Prof. Hiroshi Maeda, Department of Endodontics, Osaka Dental University, for his continuous dedication to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest regarding this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10266_2019_456_MOESM1_ESM.tiff
Supplementary Fig. 1: Structural formula of abietic acid: Terpenes consist of isoprene units with 5 carbons. Abietic acid is a diterpene derived from four isoprenes (molecular weight: 302.44)
Rights and permissions
About this article
Cite this article
Ito, Y., Ito, T., Yamashiro, K. et al. Antimicrobial and antibiofilm effects of abietic acid on cariogenic Streptococcus mutans. Odontology 108, 57–65 (2020). https://doi.org/10.1007/s10266-019-00456-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-019-00456-0