Abstract
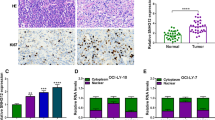
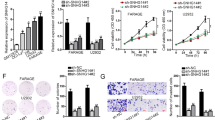
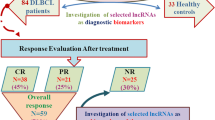
Diffuse large B cell lymphoma (DLBCL) is one of the most common malignancies worldwide. To date, there has been little progress in improving the overall survival of DLBCL patients. Emerging evidences have implicated that long noncoding RNAs (lncRNAs) have important regulatory roles in fundamental biological processes, and some of them are involved in cancer initiation, development and progression. This study was to investigate the expression of lncRNA PEG10 in a cohort of DLBCL patients to assess its clinical value and biological function in DLBCL. We first found that the expression of PEG10 was upregulated in DLBCL tumorous tissues and that cell lines compared with the normal. Moreover, we illustrated that PEG10 was significantly correlated with B symptoms, IPI score, CHOP-like treatment and rituximab. In addition, ROCAUC of PEG10 was up to 0.8228, implicating that PEG10 could be a diagnostic marker for distinguishing DLBCL from normal. Importantly, we verified that PEG10 was a key independent predictive factor for DLBCL prognosis from sizable samples through the longtime follow-ups. Furthermore, we revealed that knockdown of PEG10 expression by siRNA could lead to growth arrest and cell apoptosis in vitro. Our results suggested that PEG10 could represent a novel indicator of poor prognosis and might be served as a potential target for the diagnosis and gene therapy of DLBCL.




Similar content being viewed by others
References
Hunt KE, Reichard KK. Diffuse large B-cell lymphoma. Arch Pathol Lab Med. 2008;132(1):118–24.
Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–47.
Küppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5(4):251–62.
Shipp MA, Ross KN, Tamayo P, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8(1):68–74.
Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21(6):354–61.
Gutschner T, Hämmerle M, Eißmann M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73(3):1180–9.
Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–6.
Peng W, Gao W, Feng J. Long noncoding RNA HULC is a novel biomarker of poor prognosis in patients with pancreatic cancer. Med Oncol. 2014;31(12):1–7.
Ono R, Kobayashi S, Wagatsuma H, et al. A retrotransposon-derived gene, PEG10, is a novel imprinted gene located on human chromosome 7q21. Genomics. 2001;73(2):232–7.
Okabe H, Satoh S, Furukawa Y, et al. Involvement of PEG10 in human hepatocellular carcinogenesis through interaction with SIAH1. Cancer Res. 2003;63(12):3043–8.
Kainz B, Shehata M, Bilban M, et al. Overexpression of the paternally expressed gene 10 (PEG10) from the imprinted locus on chromosome 7q21 in high-risk B-cell chronic lymphocytic leukemia. Int J Cancer. 2007;121(9):1984–93.
Zang W, Wang T, Huang J, et al. Long noncoding RNA PEG10 regulates proliferation and invasion of esophageal cancer cells. Cancer Gene Ther. 2015;. doi:10.1038/cgt.2014.77.
Hu C, Xiong J, Zhang L et al. PEG10 activation by co-stimulation of CXCR5 and CCR7 essentially contributes to resistance to apoptosis in CD19 + CD34 + B cells from patients with B cell lineage acute and chronic lymphocytic leukemia. Cell Mol Immunol 2004;1(4):280.
Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–74.
Tsou A-P, Chuang Y-C, Su J-Y, et al. Overexpression of a novel imprinted gene, PEG10, in human hepatocellular carcinoma and in regenerating mouse livers. J Biomed Sci. 2003;10(6):625–35.
Tsuji K, Yasui K, Gen Y, et al. PEG10 is a probable target for the amplification at 7q21 detected in hepatocellular carcinoma. Cancer Genet Cytogenet. 2010;198(2):118–25.
Liu DC, Yang ZL, Jiang S. Identification of PEG10 and TSG101 as carcinogenesis, progression, and poor-prognosis related biomarkers for gallbladder adenocarcinoma. Pathol Oncol Res POR. 2011;17(4):859–66. doi:10.1007/s12253-011-9394-7.
Xiong J, Qin J, Zheng YC, Peng XF, Luo YX, Meng XY. PEG10 promotes the migration of human Burkitt’s lymphoma cells by up-regulating the expression of matrix metalloproteinase-2 and -9. Clin Investig Eff. 2012;35(3):E117.
Acknowledgments
This work was supported by Grants from Research Office of Jiangsu Cancer Hospital (No. ZK201401) and Agency of Jiangsu Province Science and Technology (No. 2013035).
Conflict of interest
None.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wei Peng and Hong Fan have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Peng, W., Fan, H., Wu, G. et al. Upregulation of long noncoding RNA PEG10 associates with poor prognosis in diffuse large B cell lymphoma with facilitating tumorigenicity. Clin Exp Med 16, 177–182 (2016). https://doi.org/10.1007/s10238-015-0350-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-015-0350-9