Abstract
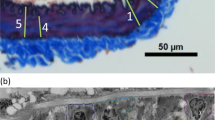
Pregnancy associates with dramatic changes in maternal cardiovascular physiology that ensure that the utero-placental circulation can support the developing fetus. Particularly striking is the marked flow-induced remodeling of uterine arteries during pregnancy and their recovery following birth. Whereas details are available in the literature on alterations in hemodynamics within and changes in the dimensions of uterine arteries during and following pregnancy in mice, we report here the first biaxial biomechanical phenotyping of these arteries during this dynamic period of growth and remodeling (G&R). To gain additional insight into the measured G&R, we also use a computational constrained mixture model to describe and predict findings, including simulations related to complications that may arise during pregnancy. It is found that dramatic pregnancy-induced remodeling of the uterine artery is largely, but not completely, reversed in the postpartum period, which appears to be driven by increases in collagen turnover among other intramural changes. By contrast, data on the remodeling of the ascending aorta, an elastic artery, reveal modest changes that are fully recovered postpartum. There is strong motivation to continue biomechanical studies on this critical aspect of women’s health, which has heretofore not received appropriate consideration from the biomechanics community.





Similar content being viewed by others
References
Allerkamp HH, Clark AR, Lee TC, Morgan TK, Burton GJ, James JL (2021) Something old, something new: digital quantification of uterine vascular remodelling and trophoblast plugging in historical collections provides new insight into adaptation of the utero-placental circulation. Hum Reprod 36:571–586
Allerkamp HH, Pole T, Boukham A, James JL, Clark AR (2022) Pregnancy-specific uterine vascular reactivity: a data-driven computational model of shear-dependent, myogenic, and mechanical radial artery features. Am J Physiol Heart Circ Physiol 323:H72–H88
Baek S, Gleason RL, Rajagopal KR, Humphrey JD (2007) Theory of small on large: potential utility in computations of fluid-solid interactions in arteries. Comp Methods Appl Mech Eng 196:3070–3078
Burton GJ, Woods AW, Jauniaux E, Kingdom JC (2009) Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30:473–482
Clark AR, James JL, Stevenson GN, Collins SL (2018) Understanding abnormal uterine artery Doppler waveforms: a novel computational model to explore potential causes within the utero-placental vasculature. Placenta 66:74–81
Dajnowiec D, Langille BL (2007) Arterial adaptations to chronic changes in haemodynamic function: coupling vasomotor tone to structural remodelling. Clin Sci (lond) 113(1):15–23
Drews JD, Pepper VK, Best CA, Szafron JM, Cheatham JP, Yates AR, Hor KN, Zbinden JC, Chang YC, Mirhaidari GJM, Ramachandra AB, Miyamoto S, Blum KM, Onwuka EA, Zakko J, Kelly J, Cheatham SL, King N, Reinhardt JW, Sugiura T, Miyachi H, Matsuzaki Y, Breuer J, Heuer ED, West TA, Shoji T, Berman D, Boe BA, Asnes J, Galantowicz M, Matsumura G, Hibino N, Marsden AL, Pober JS, Humphrey JD, Shinoka T, Breuer CK (2020) Spontaneous reversal of stenosis in tissue-engineered vascular grafts. Sci Transl Med 12(537):eaax6919
Espinoza J, Romero R, Mee Kim Y, Kusanovic JP, Hassan S, Erez O, Gotsch F, Than NG, Papp Z, Jai Kim C (2006) Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med 34(6):447–458
Ferruzzi J, Bersi MR, Humphrey JD (2013) Biomechanical phenotyping of central arteries in health and disease: advantages of and methods for murine models. Ann Biomed Eng 41:1311–1330
Gleason RL, Gray SP, Wilson E, Humphrey JD (2004) A multiaxial computer-controlled organ culture and biomechanical device for mouse carotid arteries. ASME J Biomech Eng 126:787–795
Griendling KK, Fuller EO, Cox RH (1985) Pregnancy-induced changes in sheep uterine and carotid arteries. Am J Physiol 248:H658–H665
Humphrey JD (2008) Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension 52:195–200
Humphrey JD (2021) Constrained mixture models of tissue growth and remodelling—twenty years after. J Elast 145:49–75
Humphrey JD, Rajagopal KR (2002) A constrained mixture model for growth and remodeling of soft tissues. Math Model Methods Appl Sci 12:407–430
Humphrey JD, Eberth JF, Dye WW, Gleason RL (2009) Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech 42:1–8
James JL, Saghian R, Perwick R, Clark AR (2018) Trophoblast plugs: impact on utero-placental haemodynamics and spiral artery remodelling. Hum Reprod 33(8):1430–1441
Khankin EV, Ko NL, Mandala M, Karumanchi SA, Osol G (2021) Normalization of wall shear stress as a physiological mechanism for regulating material uterine artery expansive remodeling during pregnancy. FASEB BioAdv 3:702–708
Kulandavelu S, Whiteley KJ, Qu D, Mu J, Bainbridge SA, Adamson SL (2012) Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice. Hypertension 60:231–238
Latorre M, Humphrey JD (2018a) Modeling mechano-driven and immuno-mediated aortic maladaptation in hypertension. Biomech Model Mechanobiol 17:1497–1511
Latorre M, Humphrey JD (2018b) A mechanobiologically equilibrated constrained mixture model for growth and remodeling of soft tissues. Z Angew Math Mech (ZAMM) 98:2048–2071
Lloyd-Davies C, Collins SL, Burton GJ (2021) Understanding the uterine artery Doppler waveform and its relationship to spiral artery remodelling. Placenta 105:78–84
Mu J, Adamson SL (2006) Developmental changes in hemodynamics of uterine artery, utero- and umbilico-placental, and vitelline circulations in mouse throughout gestation. Am J Physiol 291:H1421–H1428
Osol G, Mandala M (2009) Maternal uterine vascular remodeling during pregnancy. Physiology 24:58–71
Osol G, Moore LG (2014) Maternal uterine vascular remodeling during pregnancy. Microcirculation 21:38–47
Pastore MB, Jobe SO, Ramadoss J, Maqness RR (2012) Estrogen receptor-α and estrogen receptor-β in the uterine vascular endothelium during pregnancy: functional implications for regulating uterine blood flow. Semin Reprod Med 30:46–61
Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC (1998) Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 101:731–736
Sanghavi M, Rutherford JD (2014) Cardiovascular physiology of pregnancy. Circulation 130:1003–1008
Schroeder F, Polzer S, Slazansky M, Man V, Skacel P (2018) Predictive capabilities of various constitutive models for arterial tissue. J Mech Behav Biomed Mater 78:369–380
Tarhouni K, Guihot AL, Freidja ML, Toutain B, Henrion B, Baufreton C, Pinaud F, Procaccio V, Grimaud L, Ayer A, Loufrani L, Lenfant F, Arnal JF, Henrion D (2013) Key role of estrogens and endothelial estrogen receptor α in blood flow-mediated remodeling of resistance arteries. Arterioscler Thromb Vasc Biol 33:605–611
Thaler I, Manor D, Itskovitz J, Rottem S, Levit N, Tritsch-Timor I, Brandes JM (1990) Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol 162:121–125
Van Buren GA, Yang D-S, Clark KE (1992) Estrogen-induced uterine vasodilatation is antagonized by L-nitroargine metyl ester, and inhibitor of nitric oxide synthesis. Am J Obstet Gynecol 167:828–833
Weiss D, Latorre M, Rego B, Cavinato C, Berman AG, Goergen CJ, Humphrey JD (2021) Biomechanical consequences of compromised elastic fiber integrity and matrix cross-linking on abdominal aortic aneurysmal enlargement. Acta Biomat 134:422–434
Yamashiro Y, Yanagisawa H (2020) The molecular mechanism of mechanotransduction in vascular homeostasis and disease. Clin Sci (lond) 134:2399–2418
Acknowledgements
This work was made possible by discretionary funds provided by Yale University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Murtada, SI., Latorre, M. & Humphrey, J.D. Remodeling of the uterine artery during and early after pregnancy in the mouse. Biomech Model Mechanobiol 22, 1531–1540 (2023). https://doi.org/10.1007/s10237-022-01674-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-022-01674-2