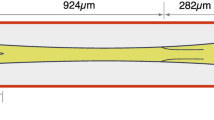
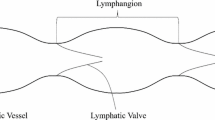
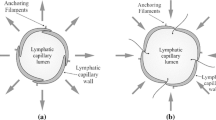
Abstract
A three-dimensional finite-element fluid/structure interaction model of an intravascular lymphatic valve was constructed, and its properties were investigated under both favourable and adverse pressure differences, simulating valve opening and valve closure, respectively. The shear modulus of the neo-Hookean material of both vascular wall and valve leaflet was varied, as was the degree of valve opening at rest. Also investigated was how the valve characteristics were affected by prior application of pressure inflating the whole valve. The characteristics were parameterised by the volume flow rate through the valve, the hydraulic resistance to flow, and the maximum sinus radius and inter-leaflet-tip gap on the plane of symmetry bisecting the leaflet, all as functions of the applied pressure difference. Maximum sinus radius on the leaflet-bisection plane increased with increasing pressure applied to either end of the valve segment, but also reflected the non-circular deformation of the sinus cross section caused by the leaflet, such that it passed through a minimum at small favourable pressure differences. When the wall was stiff, the inter-leaflet gap increased sigmoidally during valve opening; when it was as flexible as the leaflet, the gap increased more linearly. Less pressure difference was required both to open and to close the valve when either the wall or the leaflet material was more flexible. The degree of bias of the valve characteristics to the open position increased with the inter-leaflet gap in the resting position and with valve inflation pressure. The characteristics of the simulated valve were compared with those specified in an existing lumped-parameter model of one or more collecting lymphangions and used to estimate a revised value for the constant in that model which controls the rate of valve opening/closure with variation in applied pressure difference. The effects of the revised value on the lymph pumping efficacy predicted by the lumped-parameter model were evaluated.












Similar content being viewed by others
Notes
Blatter et al. (2017) have observed mouse lymphatic valves to open in less than one unit and close in less than two units of 0.28 s, this being the limit of time resolution of their technique. The 30 frame/s videos from the Davis lab show that opening and closing times vary greatly with factors controlling the rate at which the transvalvular pressure difference changes (personal observation, CDB). Such factors include among others the prevailing adverse pressure difference for the vessel, the transmural pressure, and the vigour of contractions.
The maxima of retrograde flow rate were − 4.4, − 8.8 and − 17.3 μL/hr for inflation pressure 0, 0.5 and 1 cm H2O respectively. They occurred at pressure differences − 53, − 87 and − 125 dyn/cm2, when the half-gaps were 2.74, 3.52 and 4.62 μm respectively.
In the lumped-parameter valve model with transmural-pressure-dependent bias (Bertram et al. 2014b), ∆po is not constant, but varies between approximately 0 and ‒ 2210 dyn/cm2.
If this same degree of valve characteristic offset is introduced with so taking its accustomed value of 0.2 cm2/dyn, the result (with all other parameters and boundary conditions unchanged) is that V1 stays open and V2 stays shut all the time, and there is constant slight leakage backflow (0.9 μL/hr) past the closed valve, propelled by the 2.5 cm H2O adverse pressure drop. Contraction produces only an ineffectual to-and-fro sloshing past the open inlet valve.
Unpublished observations by M.J. Davis suggest that, while there is much variation between individual valves, murine valve closure tends to be a stable, fully reversible process at low transmural pressure, only becoming unstable at higher transmural pressure.
In a simulation for the 10 μm half-gap model with Gleaflet = 20 kPa and Gwall = 2000 kPa (not shown), closure required an adverse pressure difference of over 1000 dyn/cm2.
References
Ballard M, Wolf KT, Nepiyushchikh Z, Dixon JB, Alexeev A (2018) Probing the effect of morphology on lymphatic valve dynamic function. Biomech Model Mechanobiol 17(5):1343–1356. https://doi.org/10.1007/s10237-018-1030-y
Bertram CD, Macaskill C, Davis MJ, Moore JE Jr (2014a) Development of a model of a multi-lymphangion lymphatic vessel incorporating realistic and measured parameter values. Biomech Model Mechanobiol 13(2):401–416. https://doi.org/10.1007/s10237-013-0505-0
Bertram CD, Macaskill C, Davis MJ, Moore JE Jr (2017) Valve-related modes of pump failure in collecting lymphatics: numerical and experimental investigation. Biomech Model Mechanobiol 16(6):1987–2003. https://doi.org/10.1007/s10237-017-0933-3
Bertram CD, Macaskill C, Davis MJ, Moore JE Jr (2018) Contraction of collecting lymphatics: organization of pressure-dependent rate for multiple lymphangions. Biomech Model Mechanobiol 17(5):1513–1532. https://doi.org/10.1007/s10237-018-1042-7
Bertram CD, Macaskill C, Moore JE Jr (2011) Simulation of a chain of collapsible contracting lymphangions with progressive valve closure. ASME J Biomech Eng 133(1):011008-1–011008-10. https://doi.org/10.1115/1.4002799
Bertram CD, Macaskill C, Moore JE Jr (2014b) Incorporating measured valve properties into a numerical model of a lymphatic vessel. Comput Methods Biomech Biomed Eng 17(14):1519–1534. https://doi.org/10.1080/10255842.2012.753066
Blatter C, Meijer EFJ, Nam AS, Jones D, Bouma BE, Padera TP, Vakoc BJ (2016) In vivo label-free measurement of lymph flow velocity and volumetric flow rates using Doppler optical coherence tomography. Sci Rep 6:29035-1–29035-10. https://doi.org/10.1038/srep29035
Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D (2009) Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol 297(4):H1319–H1328. https://doi.org/10.1152/ajpheart.00039.2009
Bridenbaugh EA, Nizamutdinova IT, Jupiter D, Nagai T, Thangaswamy S, Chatterjee V, Gashev AA (2013) Lymphatic muscle cells in rat mesenteric lymphatic vessels of various ages. Lymphat Res Biol 11(1):35–42. https://doi.org/10.1089/lrb.2012.0025
Carew TE, Vaishnav RN, Patel DJ (1968) Compressibility of the arterial wall. Circ Res 23:61–68
Contarino C, Toro EF (2018) A one-dimensional mathematical model of collecting lymphatics coupled with an electro-fluid-mechanical contraction model and valve dynamics. Biomech Model Mechanobiol 17(6):1687–1714. https://doi.org/10.1007/s10237-018-1050-7
Davis MJ, Rahbar E, Gashev AA, Zawieja DC, Moore JE Jr (2011) Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol 301(1):H48–H60. https://doi.org/10.1152/ajpheart.00133.2011
Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE Jr, Zawieja DC (2006) Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13(7):597–610. https://doi.org/10.1080/10739680600893909
Kunert C, Baish JW, Liao S, Padera TP, Munn LL (2015) Mechanobiological oscillators control lymph flow. Proc Nat Acad Sci USA 112(35):10938–10943. https://doi.org/10.1073/pnas.1508330112
Lapinski PE, Lubeck BA, Chen D, Doosti A, Zawieja SD, Davis MJ, King PD (2017) RASA1 regulates the function of lymphatic vessel valves in mice. J Clin Invest 127(7):2569–2585. https://doi.org/10.1172/JCI89607
Li H, Mei Y, Maimon N, Padera TP, Baish JW, Munn LL (2019) The effects of valve leaflet mechanics on lymphatic pumping assessed using numerical simulations. Sci Rep 9:10649-1–10649-17. https://doi.org/10.1038/s41598-019-46669-9
Macdonald AJ (2008) The Computational Modelling of Collecting Lymphatic Vessels. Ph.D, University of Exeter, Exeter, UK
Margaris KN, Nepiyushchikh Z, Zawieja DC, Moore J Jr, Black RA (2016) Microparticle image velocimetry approach to flow measurements in isolated contracting lymphatic vessels. Journal of Biomedical Optics 21 (2):025002-1–025002-11. https://doi.org/10.1117/1.JBO.21.2.025002
Mazzoni MC, Skalak TC, Schmid-Schönbein GW (1987) Structure of lymphatic valves in the spinotrapezius muscle of the rat. Blood Vessels 24(6):304–312
Moore JE Jr, Bertram CD (2018) Lymphatic system flows. Annu Rev Fluid Mech 50:459–482. https://doi.org/10.1146/annurev-fluid-122316-045259
Phillips MN, Jones GT, van Rij AM, Zhang M (2004) Micro-venous valves in the superficial veins of the human lower limb. Clin Anat 17:55–60. https://doi.org/10.1002/ca.10141
Rahbar E, Weimer J, Gibbs H, Yeh AT, Bertram CD, Davis MJ, Hill MA, Zawieja DC, Moore JE Jr (2012) Passive pressure-diameter relationship and structural composition of rat mesenteric lymphangions. Lymphat Res Biol 10(4):152–163. https://doi.org/10.1089/lrb.2011.0015
Reddy NP, Krouskop TA, Newell PH Jr (1975) Biomechanics of a lymphatic vessel. Blood Vessels 12(5):261–278 (correction publ 14(2):128)
Sabine A, Petrova TTV (2014) Interplay of mechanotransduction, FOXC2, connexins, and calcineurin signaling in lymphatic valve formation. In: Kiefer F, Schulte-Merker S (eds) Developmental aspects of the lymphatic vascular system. Advances in anatomy, embryology and cell biology, vol 214. Springer, Wien, pp 67–80. https://doi.org/10.1007/978-3-7091-1646-3_6
Telinius N, Drewsen N, Pilegaard H, Kold-P etersen H, de Leval M, Aalkjaer C, Hjortdal V, Boedtkjer DB (2010) Human thoracic duct in vitro: diameter-tension properties, spontaneous and evoked contractile activity. Am J Physiol (Heart Circ Physiol) 299(3):H811–H818. https://doi.org/10.1152/ajpheart.01089.2009
Venugopal AM, Stewart RH, Laine GA, Dongaonkar RM, Quick CM (2007) Lymphangion coordination minimally affects mean flow in lymphatic vessels. American Journal of Physiology - Heart and Circulatory Physiology 293(2):H1183–H1189. https://doi.org/10.1152/ajpheart.01340.2006
Wilson JT, Edgar LT, Prabhakar S, Horner M, van Loon R, Moore JE Jr (2018) A fully coupled fluid-structure interaction model of the secondary lymphatic valve. Comput Methods Biomech Biomed Eng 21(16):813–823. https://doi.org/10.1080/10255842.2018.1521964
Wilson JT, van Loon R, Wang W, Zawieja DC, Moore JE Jr (2015) Determining the combined effect of the lymphatic valve leaflets and sinus on resistance to forward flow. J Biomech 48(13):3584–3590. https://doi.org/10.1016/j.jbiomech.2015.07.045
Wilson JT, Wang W, Hellerstedt AH, Zawieja DC, Moore Jr JE (2013) Confocal image-based computational modeling of nitric oxide transport in a rat mesenteric lymphatic vessel. ASME J Biomech Eng 135(5):051005-1–051005-8. https://doi.org/10.1115/1.4023986 [erratum publ 135(11), 117001. https://doi.org/10.1115/1.4025336]
Zawieja DC (2009) Contractile physiology of lymphatics. Lymphat Res Biol 7(2):87–96. https://doi.org/10.1089/lrb.2009.0007
Zawieja SD, Castorena-Gonzalez JA, Scallan JP, Davis MJ (2018) Differences in L-type Ca2+ channel activity partially underlie the regional dichotomy in pumping behavior by murine peripheral and visceral lymphatic vessels. Am J Physiol Heart Circ Physiol 314(5):H991–H1010. https://doi.org/10.1152/ajpheart.00499.2017
Acknowledgements
Particularly excellent and essential help and advice were given by Mr. Nicholas Medeiros, a former support engineer at ADINA R&D Inc., as well as assistance from other ADINA support staff. I acknowledge extensive discussions with Associate Professor Charlie Macaskill through the course of this work. I thank Professors Michael J. Davis and James E. Moore Jr. and Associate Professor Raoul van Loon for valuable discussions, and three anonymous reviewers for useful suggestions. I acknowledge funding from NIH Grant U01-HL-123420.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Equations of the model
where p1, pm, and p2 = pressure at the upstream end, centre, and downstream end of the lymphangion, pa and pb = upstream and downstream reservoir pressures, p0 = pressure upstream of the inlet valve but downstream of the inlet cannula, and pp = pressure downstream of the outlet valve but upstream of the outlet cannula; Q1 and Q2 = flow-rates into and out of the lymphangion, and D = lymphangion diameter. The resistances of the inlet and outlet valves, RV1 and RV2, are given by
where ∆pV1 = p0 − p1, ∆pV2 = p2 − pp, the open/close threshold ∆po1 depends on either p0 − pe or p1 − pe according to the current valve state, and ∆po2 depends on either p2 − pe or pp − pe (Bertram et al. 2014a). The constants in eqns. A1 − 4 are the lymphangion length L (0.3 cm), the lymph viscosity µ (1 cP), the resistances of the inlet and outlet cannulae (Ra and Rb, respectively), the external pressure pe, and RVn, RVx and so as defined in the main text.
Constitutive relation Two curves fitted to twitch-contraction data [see Bertram et al. (2017)] describe the maximally active and passive states as
where subscripts act and psv indicate the peak-twitch and pre-twitch states, respectively.
Time course of active contraction:
where the activation waveform M(t) is calculated in two steps; in the first,
where fm = mf, tc is the beginning of a contraction, and 1/f is its duration. A function Mj(t) modifies the preliminary M(t) to its final form, where
Constant m multiplies the rate of onset and decay of the active state; with m = 1, M(t) is a sinusoid.
Pressure-dependent diastole The duration of the period of relaxation tr that starts at t = tc + 1/f is
where \(f_{{{\text{tw}}}} = 60\left( { - 1.39q^{2} + 12.6q + 0.647} \right)\), with \( q = {\ln}\left( {1 + {\overline {\Delta {p}}}_{{{\text{tm}}}} } \right)\), \( {\overline {\Delta {p}}}_{{{\text{tm}}}}\) being the average transmural pressure over the immediately preceding systole, defined as
where ∆ptm = pm − pe (in cm H2O).
Valve characteristics The twin curves that determine ∆po (Eqs. 4 and 8) for opening and closure of each valve are shown in Fig.
Variation of ∆po with valve transmural pressure. Solid black and magenta: curves fitted to data measured by Davis et al. (2011) for opening and closing, respectively. Broken magenta: closure curve, times constant cfact (Bertram et al. 2014b). Solid and broken green: opening and closing characteristics, respectively, offset by − 0.6 cm H2O as for right-hand panels of Fig. 12. Pressure pint = pin for opening, pout for closure
13.
Rights and permissions
About this article
Cite this article
Bertram, C.D. Modelling secondary lymphatic valves with a flexible vessel wall: how geometry and material properties combine to provide function. Biomech Model Mechanobiol 19, 2081–2098 (2020). https://doi.org/10.1007/s10237-020-01325-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-020-01325-4