Abstract
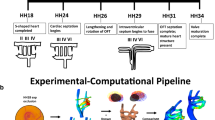
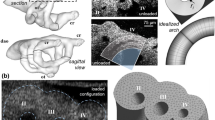
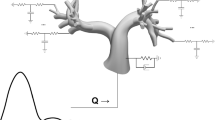
The majority of severe clinically significant forms of congenital heart disease (CHD) are associated with great artery lesions, including hypoplastic, double, right or interrupted aortic arch morphologies. While fetal and neonatal interventions are advancing, their potential ability to restore cardiac function, optimal timing, location, and intensity required for intervention remain largely unknown. Here, we combine computational fluid dynamics (CFD) simulations with in vivo experiments to test how individual pharyngeal arch artery hemodynamics alter as a result of local interventions obstructing individual arch artery flow. Simulated isolated occlusions within each pharyngeal arch artery were created with image-derived three-dimensional (3D) reconstructions of normal chick pharyngeal arch anatomy at Hamburger–Hamilton (HH) developmental stages HH18 and HH24. Acute flow redistributions were then computed using in vivo measured subject-specific aortic sinus inflow velocity profiles. A kinematic vascular growth-rendering algorithm was then developed and implemented to test the role of changing local wall shear stress patterns in downstream 3D morphogenesis of arch arteries. CFD simulations predicted that altered pressure gradients and flow redistributions were most sensitive to occlusion of the IVth arches. To evaluate these simulations experimentally, a novel in vivo experimental model of pharyngeal arch occlusion was developed and implemented using two-photon microscopy-guided femtosecond laser-based photodisruption surgery. The right IVth arch was occluded at HH18, and resulting diameter changes were followed for up to 24 h. Pharyngeal arch diameter responses to acute hemodynamic changes were predicted qualitatively but poorly quantitatively. Chronic growth and adaptation to hemodynamic changes, however, were predicted in a subset of arches. Our findings suggest that this complex biodynamic process is governed through more complex forms of mechanobiological vascular growth rules. Other factors in addition to wall shear stress or more complex WSS rules are likely important in the long-term arterial growth and patterning. Combination in silico/experimental platforms are essential for accelerating our understanding and prediction of consequences from embryonic/fetal cardiovascular occlusions and lay the foundation for noninvasive methods to guide CHD diagnosis and fetal intervention.







Similar content being viewed by others
References
Anderson DA, Tannehill JC, Pletcher RH (1984) Computational fluid mechanics and heat transfer. Hemisphere Publishing Corporation, New York
Bajolle F, Zaffran S, Kelly RG, Hadchouel J, Bonnet D, Brown NA, Buckingham ME (2006) Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ Res 98:421–428
Bayer IM, Adamson SL, Langille BL (1999) Atrophic remodeling of the artery-cuffed artery. Arterioscler Thromb Vasc Biol 19:1499–1505
Bergwerff M et al (1996) Onset of elastogenesis and downregulation of smooth muscle actin as distinguishing phenomena in artery differentiation in the chick embryo. Anat Embryol 194(6):545–557
Bharadwaj KN, Spitz C, Shekhar A, Yalcin HC, Butcher JT (2012) Computational fluid dynamics of developing avian outflow tract heart valves. Ann Biomed Eng 40:2212–2227
Bockman DE, Redmond ME, Kirby ML (1989) Alteration of early vascular development after ablation of cranial neural crest. Anat Rec 225(3):209–217. doi:10.1002/ar.1092250306
Bremer JL (1928) Experiments on the aortic arches in the chick. Anat Rec (Hoboken) 37:225–254
Broekhuizen ML, Mast F, Struijk PC, van der Bie W, Mulder PG, Gittenberger-de Groot AC, Wladimiroff JW (1993) Hemodynamic parameters of stage 20 to stage 35 chick embryo. Pediatr Res 34:44–46
Butcher JT, Sedmera D, Guldberg RE, Markwald RR (2007) Quantitative volumetric analysis of cardiac morphogenesis assessed through micro-computed tomography. Dev Dyn 236:802–809
Clark EB, Hu N, Frommelt P, Vandekieft GK, Dummett JL, Tomanek RJ (1989) Effect of increased pressure on ventricular growth in stage 21 chick embryos. Am J Physiol 257:H55–H61
Culver JC, Dickinson ME (2010) The effects of hemodynamic force on embryonic development. Microcirculation 17:164–178
Davis JC (1986) Statistics and data analysis in geology, 2nd edn. John Wiley, New York
deAlmeida A, McQuinn T, Sedmera D (2007) Increased ventricular preload is compensated by myocyte proliferation in normal and hypoplastic fetal chick left ventricle. Circ Res 100:1363–1370
Dor X, Corone P (1985) Migration and torsions of the conotruncus in the chick embryo heart: observational evidence and conclusions drawn from experimental intervention. Heart Vessels 1:195–211
Elzenga NJ, Gittenberger-de Groot AC (1985) Coarctation and related aortic arch anomalies in hypoplastic left heart syndrome. Int J Cardiol 8:379–393
Emery SP, Kreutzer J, Sherman FR, Fujimoto KL, Jaramaz B, Nikou C, Tobita K, Keller BB (2007) Computer-assisted navigation applied to fetal cardiac intervention. Int J Med Robot 3:187–198
Figueroa CA, Baek S, Taylor CA, Humphrey JD (2009) A computational framework for fluid-solid-growth modeling in cardiovascular simulations. Comput Methods Appl Mech Eng 198:3583–3602
Gessner IH (1966a) Spectrum of congenital cardiac anomalies produced in chick embryos by mechanical interference with cardiogenesis. Circ Res 18:625–633
Gessner IH (1966b) Spectrum of congenital cardiac anomalies produced in chick embryos by mechanical interference with cardiogenesis. Circ Res 18:625–633
Girerd X, London G, Boutouyrie P, Mourad JJ, Safar M, Laurent S (1996) Remodeling of the radial artery in response to a chronic increase in shear stress. Hypertension 27:799–803
Go AS et al (2013) Executive summary: heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation 127(1):143–152
Graham A (2003) Development of the pharyngeal arches. Am J Med Genet A 119A:251–256
Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE (1999) Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal. Cardiovasc Res 41:87–99
Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M (2003) Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421:172–177
Hu N, Clark EB (1989) Hemodynamics of the stage 12 to stage 29 chick embryo. Circ Res 65:1665–1670
Hu N, Christensen DA, Agrawal AK, Beaumont C, Clark EB, Hawkins JA (2009) Dependence of aortic arch morphogenesis on intracardiac blood flow in the left atrial ligated chick embryo. Anatomical record 292:652–660
Huang C, Sheikh F, Hollander M, Cai C, Becker D, Chu PH, Evans S, Chen J (2003) Embryonic atrial function is essential for mouse embryogenesis, cardiac morphogenesis and angiogenesis. Development 130:6111–6119
Humphrey JD, Rajagopal KR (2002) A constrained mixture model for growth and remodeling of soft tissues. Math Mod Meth Appl Sci 12:407–430
Ilbawi AM et al (2007) Morphologic study of the ascending aorta and aortic arch in hypoplastic left hearts: surgical implications. J Thorac Cardiovasc Surg 134(1):99–105
Jaffee OC (1965) Hemodynamic factors in the development of the chick embryo heart. Anat Rec 151:69–75
Kamiya A, Togawa T (1980) Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol 239:H14–21
Kirby M (2002) Molecular embryogenesis of the heart. Pediatr Dev Pathol 5:516–543
Kirby ML, Hunt P, Wallis K, Thorogood P (1997) Abnormal patterning of the aortic arch arteries does not evoke cardiac malformations. Dev Dyn 208:34–47
Kowalski WJ, Teslovich NC, Dur O, Keller BB, Pekkan K (2012) Computational hemodynamic optimization predicts dominant aortic arch selection is driven by embryonic outflow tract orientation in the chick embryo. Biomech Model Mechanobiol 11:1057–1073
Kowalski WJ, Dur O, Wang Y, Patrick MJ, Tinney JP, Keller BB, Pekkan K (2013) Critical transitions in early embryonic aortic arch patterning and hemodynamics. PloS One 8:e60271
Langille BL (1996) Arterial remodeling: relation to hemodynamics. Can J Physiol Pharmacol 74:834–841
Langille BL, O’Donnell F (1986) Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231:405–407
Le Lièvre CS, Le Douarin NM (1975) Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol 34(1):125–154
le Noble F, Fleury V, Pries A, Corvol P, Eichmann A, Reneman RS (2005) Control of arterial branching morphogenesis in embryogenesis: go with the flow. Cardiovasc Res 65:619–628
Liu C, Liu W, Palie J, Lu MF, Brown NA, Martin JF (2002) Pitx2c patterns anterior myocardium and aortic arch vessels and is required for local cell movement into atrioventricular cushions. Development 129:5081–5091
Lucitti JL, Tobita K, Keller BB (2005) Arterial hemodynamics and mechanical properties after circulatory intervention in the chick embryo. J Exp Biol 208:1877–1885
Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME (2007) Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134:3317–3326
Macatee TL, Hammond BP, Arenkiel BR, Francis L, Frank DU, Moon AM (2003) Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development 130:6361–6374
May SR, Stewart NJ, Chang W, Peterson AS (2004) A Titin mutation defines roles for circulation in endothelial morphogenesis. Dev Biol 270:31–46
McElhinney DB, Tworetzky W, Lock JE (2010) Current status of fetal cardiac intervention. Circulation 121:1256–1263
Menon PG, Teslovich N, Chen CY, Undar A, Pekkan K (2013) Characterization of neonatal aortic cannula jet flow regimes for improved cardiopulmonary bypass. J Biomech 46:362–372
Molin DG, DeRuiter MC, Wisse LJ, Azhar M, Doetschman T, Poelmann RE, Gittenberger-de Groot AC (2002) Altered apoptosis pattern during pharyngeal arch artery remodelling is associated with aortic arch malformations in Tgfbeta2 knock-out mice. Cardiovasc Res 56:312–322
Molin DG, Poelmann RE, DeRuiter MC, Azhar M, Doetschman T, Gittenberger-de Groot AC (2004) Transforming growth factor beta-SMAD2 signaling regulates aortic arch innervation and development. Circ Res 95:1109–1117
Nishimura N et al (2006) Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke. Nat Methods 3(2):99–108
Pexieder T (1986) Standardized method for study of normal and abnormal cardiac development in chick, rat, mouse, dog and human embryos. Teratology 33:91C–92C
Pologruto TA, Sabatini BL, Svoboda K (2003) ScanImage: flexible software for operating laser scanning microscopes. Biomed Eng Online 2–13
Reckova M, Rosengarten C, deAlmeida A, Stanley CP, Wessels A, Gourdie RG, Thompson RP, Sedmera D (2003) Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circ Res 93:77–85
Rodbard S (1975) Vascular caliber. Cardiology 60:4–49
Roman BL, Pekkan K (2012) Mechanotransduction in embryonic vascular development. Biomech Model Mechanobiol 11:1149–1168
Rosenquist TH, Beall AC (1990) Elastogenic Cells in the Developing Cardiovascular System. Ann N Y Acad Sci 588(1 Embryonic Ori), 106–119. doi:10.1111/j.1749-6632.1990.tb13201.x
Rosenquist TH, McCoy JR, Waldo KL, Kirby ML (1988) Origin and propagation of elastogenesis in the developing cardiovascular system. Anat Rec 221(4):860–871. doi:10.1002/ar.1092210411
Rychter Z (1962) Experimental morphology of the aortic arches and the heart loop in chick embryos. In: Abercrombie M, Brachet J (eds) Advances in morphogenesis. Academic Press, New York, pp 333–371
Rychter Z, Lemez L (1965) Changes in localization in aortic arches of laminar blood streams of main venous trunks to heart after exclusion of vitelline vessels on second day of incubation. Fed Proc Transl Suppl 24:815–820
Sedmera D, Pexieder T, Rychterova V, Hu N, Clark EB (1999) Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat Rec 254:238–252
Taber LA (1998) A model for aortic growth based on fluid shear and fiber stresses. J Biomech Eng 120:348–354
Taber LA, Eggers DW (1996) Theoretical study of stress-modulated growth in the aorta. J Theor Biol 180:343–357
Thoma R (1893) Untersuchungen über die Histogenese und Histomechanik des Gefässsystems. Verlag von Ferdinand Enke, Stuttgart
Tobita K, Garrison JB, Liu LJ, Tinney JP, Keller BB (2005) Three-dimensional myofiber architecture of the embryonic left ventricle during normal development and altered mechanical loads. Anat Rec A Discov Mol Cell Evol Biol 283:193–201
Ursem NT, Struijk PC, Poelmann RE, Wladimiroff JW (2001) Dorsal aortic flow velocity in chick embryos of stage 16 to 28. Ultrasound Med Biol 27:919–924
Valentin A, Humphrey JD, Holzapfel GA (2011) A multi-layered computational model of coupled elastin degradation, vasoactive dysfunction, and collagenous stiffening in aortic aging. Ann Biomed Eng 39:2027–2045
Vogel A, Venugopalan V (2003) Mechanisms of pulsed laser ablation of biological tissues. Chem Rev 103(2):577–644
Wagenseil JE (2010) A constrained mixture model for developing mouse aorta. Biomech Model Mechanobiol 10(5):671–87. doi:10.1007/s10237-010-0265-z
Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, Kumiski D, Abu-Issa R, Kirby ML (2005) Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol 281:78–90
Waldo KL, Kirby ML (1993) Cardiac neural crest contribution to the pulmonary artery and sixth aortic arch artery complex in chick embryos aged 6 to 18 days. Anat Rec 237:385–399
Waldo KL, Kumiski D, Kirby ML (1996) Cardiac neural crest is essential for the persistence rather than the formation of an arch artery. Dev Dyn 205:281–292
Wang Y, Dur O, Patrick MJ, Tinney JP, Tobita K, Keller BB, Pekkan K (2009) Aortic arch morphogenesis and flow modeling in the chick embryo. Ann Biomed Eng 37:1069–1081
Yalcin HC, Shekhar A, Nishimura N, Rane AA, Schaffer CB, Butcher JT (2010a) Two-photon microscopy-guided femtosecond-laser photoablation of avian cardiogenesis: noninvasive creation of localized heart defects. Am J Physiol Heart Circ Physiol 299:H1728–1735
Yalcin HC, Shekhar A, Rane AA, Butcher JT (2010b) An ex-ovo chicken embryo culture system suitable for imaging and microsurgery applications. J Vis Exp (44):2154. doi:10.3791/2154
Yashiro K, Shiratori H, Hamada H (2007) Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature 450:285–288
Yoshigi M, Knott GD, Keller BB (2000) Lumped parameter estimation for the embryonic chick vascular system: a time-domain approach using MLAB. Comput Methods Programs Biomed 63:29–41
Acknowledgments
This research was supported by the National Institutes of Health (HL110328 to JTB), National Science Foundation (CBET-0955712 to JTB, graduate research fellowship to SEL) and European Research Council ERC STR Grant—Vascular Growth Project No. 307460 to KP.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This collaborative study is accomplished by joint lead authors (Stephanie E. Lindsey and Prahlad G. Menon) and by joint corresponding senior authors (Jonathan T. Butcher and Kerem Pekkan).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lindsey, S.E., Menon, P.G., Kowalski, W.J. et al. Growth and hemodynamics after early embryonic aortic arch occlusion. Biomech Model Mechanobiol 14, 735–751 (2015). https://doi.org/10.1007/s10237-014-0633-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-014-0633-1