Abstract
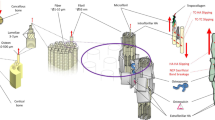
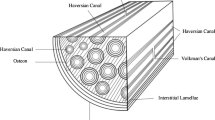
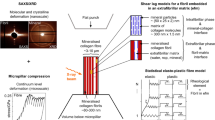
Bone, because of its hierarchical composite structure, exhibits an excellent combination of stiffness and toughness, which is due substantially to the structural order and deformation at the smaller length scales. Here, we focus on the mineralized collagen fibril, consisting of hydroxyapatite plates with nanometric dimensions aligned within a protein matrix, and emphasize the relationship between the structure and elastic properties of a mineralized collagen fibril. We create two- and three-dimensional representative volume elements to represent the structure of the fibril and evaluate the importance of the parameters defining its structure and properties of the constituent mineral and collagen phase. Elastic stiffnesses are calculated by the finite element method and compared with experimental data obtained by synchrotron X-ray diffraction. The computational results match the experimental data well, and provide insight into the role of the phases and morphology on the elastic deformation characteristics. Also, the effects of water, imperfections in the mineral phase and mineral content outside the mineralized collagen fibril upon its elastic properties are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Akiva U, Wagner HD, Weiner S (1998) Modelling the three-dimensional elastic constants of parallel-fibred and lamellar bone. J Mater Sci 33: 1497–1509
Almer JD, Stock SR (2007) Micromechanical response of mineral and collagen phases in bone. J Struct Biol 157: 365–370. doi:10.1016/j.jsb.2006.09.001
Ashby MF (1993) Criteria for selecting the components of composites. Acta Metall Mater 41: 1313–1335
Bertinetti L, Tampieri A, Landi E, Ducati C, Midgley PA, Coluccia S, Martra G (2007) Surface structure, hydration, and cationic sites of nanohydroxyapatite: UHR-TEM, IR, and microgravimetric studies. J Phys Chem C 111: 4027–4035. doi:10.1021/jp066040s
Birk DE, Silver FH, Trelstad RL (1991) Matrix Assembly. In: Hay ED (eds) Cell biology of extracellular matrix. Plenum, New York, pp 221–254
Borah B, Gross GJ, Dufresne TE, Smith TS, Cockman MD, Chmielewski PA, Lundy MW, Hartke JR, Sod EW (2001) Three-dimensional microimaging (MR mu I and mu CT), finite element modeling, and rapid prototyping provide unique insights into bone architecture in osteoporosis. Anat Rec 265: 101–110
Chen CQ, Shi Y, Zhang YS, Zhu J, Yan YJ (2006) Size dependence of Young’s modulus in ZnO nanowires. Phys Rev Lett 96: 075505. doi:10.1103/PhysRevLett.96.075505
Courtney TH (2000) Mechanical behavior of materials, 2edn. McGraw Hill, Boston
Craig AS, Birtles MJ, Conway JF, Parry DAD (1989) An estimate of the mean length of collagen fibrils in rat tail-tendon as a function of age. Connect Tissue Res 19: 51–62
Cuenot S, Fretigny C, Demoustier-Champagne S, Nysten B (2004) Surface tension effect on the mechanical properties of nanomaterials measured by atomic force microscopy. Phys Rev B 69: 165410. doi:10.1103/PhysRevB.69.165410
Currey JD (2003) Role of collagen and other organics in the mechanical properties of bone. Osteoporos Int 14: S29–S36. doi:10.1007/s00198-003-1470-8
Delmas PD, Tracy RP, Riggs BL, Mann KG (1984) Identification of the noncollagenous proteins of bovine bone by two-dimensional gel-electrophoresis. Calcif Tissue Int 36: 308–316
Dorozhkin SV, Epple M (2002) Biological and medical significance of calcium phosphates. Angew Chem Int Edit 41: 3130–3146
Fels IG (1964) Hydration and density of collagen and gelatin. J Appl Polym Sci 8: 1813–1824
Fratzl P (2003) Cellulose and collagen: from fibres to tissues. Curr Opin Colloid Interface Sci 8: 32–39. doi:10.1016/S1359-0294(03)00011-6
Fratzl P, Gupta HS, Paschalis EP, Roschger P (2004) Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem 14: 2115–2123. doi:10.1039/b402005g
Fratzl P, Weinkamer R (2007) Nature’s hierarchical materials. Prog Mater Sci 52: 1263–1334. doi:10.1016/j.pmatsci.2007.06.001
Gao HJ, Ji BH, Jäger IL, Artz E, Fratzl P (2003) Materials become insensitive to flaws at nanoscale: lessons from nature. Proc Natl Acad Sci USA 100: 5597–5600. doi:10.1073/pnas.0631609100
Gilmore RS, Katz JL (1982) Elastic properties of apatites. J Mater Sci 17: 1131–1141
Gong H, Zhang M, Qin L, Hou YJ (2007) Regional variations in the apparent and tissue-level mechanical parameters of vertebral trabecular bone with aging using micro-finite element analysis. Ann Biomed Eng 35: 1622–1631. doi:10.1007/s10439-007-9332-8
Gupta HS, Schratter S, Tesch W, Roschger P, Berzlanovich A, Schoeberl T, Klaushofer K, Fratzl P (2005) Two different correlations between nanoindentation modulus and mineral content in the bone-cartilage interface. J Struct Biol 149: 138–148. doi:10.1016/j.jsb.2004.10.010
Gupta HS, Seto J, Wagermaier W, Zaslansky P, Boesecke P, Fratzl P (2006) Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc Natl Acad Sci USA 103: 17741–17746. doi:10.1073/pnas.0604237103
Gupta HS, Wagermaier W, Zickler GA, Aroush DRB, Funari SS, Roschger P, Wagner HD, Fratzl P (2005) Nanoscale deformation mechanisms in bone. Nano Lett 5: 2108–2111. doi:10.1021/nl051584b
Hassenkam T, Fantner GE, Cutroni JA, Weaver JC, Morse DE, Hansma PK (2004) High-resolution AFM imaging of intact and fractured trabecular bone. Bone 35: 4–10. doi:10.1016/j.bone.2004.02.024
Heim AJ, Matthews WG, Koob TJ (2006) Determination of the elastic modulus of native collagen fibrils via radial indentation. Appl Phys Lett 89: 181902. doi:10.1063/1.2367660
Hoffmann U, Kwait DC, Handwerker J, Chan R, Lamuraglia G, Brady TJ (2003) Vascular calcification in ex vivo carotid specimens: precision and accuracy of measurements with multi-detector row CT. Radiology 229: 375–381. doi:10.1148/radiol.2292021016
Hulmes DJS (2002) Building collagen molecules, fibrils, and suprafibrillar structures. J Struct Biol 137: 2–10. doi:10.1006/jsbi.2002.4450
Hulmes DJS, Wess TJ, Prockop DJ, Fratzl P (1995) Radial packing, order, and disorder in collagen fibrils. Biophys J 68: 1661–1670
Jäger I, Fratzl P (2000) Mineralized collagen fibrils: a mechanical model with a staggered arrangement of mineral particles. Biophys J 79: 1737–1746
Jokanovic V, Izvonar D, Dramicanin MD, Jokanovic B, Zivojinovic V, Markovic D, Dacic B (2006) Hydrothermal synthesis and nanostructure of carbonated calcium hydroxyapatite. J Mater Sci Mater Med 17: 539–546. doi:10.1007/s10856-006-8937-z
Knowles TP, Fitzpatrick AW, Meehan S, Mott HR, Vendruscolo M, Dobson CM, Welland ME (2007) Role of intermolecular forces in defining material properties of protein nanofibrils. Science 318: 1900–1903. doi:10.1126/science.1150057
Koch CF, Johnson S, Kumar D, Jelinek M, Chrisey DB, Doraiswamy A, Jin C, Narayan RJ, Millailescu IN (2007) Pulsed laser deposition of hydroxyapatite thin films. Mater Sci Eng C Biomimetic Supramol Syst 27: 484–494. doi:10.1016/j.msec.2006.05.025
Landis WJ, Hodgens KJ, Arena J, Song MJ, McEwen BF (1996) Structural relations between collagen and mineral in bone as determined by high voltage electron microscopic tomography. Microsc Res Tech 33: 192–202
Landis WJ, Librizzi JJ, Dunn MG, Silver FH (1995) A study of the relationship between mineral content and mechanical properties of turkey gastrocnemius tendon. J Bone Miner Res 10: 859–867
Lowenstam HA, Weiner S (1989) On Biomineralization. Oxford University, New York
Lozano LF, Pena-Rico MA, Heredia A, Octolan-Flores J, Gomez- Cortes A, Velazquez R, Belio IA, Bucio L (2003) Thermal analysis study of human bone. J Mater Sci 38: 4777–4782
Martin RB, Burr DB, Sharkey NA (1998) Skeletal tissue mechanics. Springer, New York
Meyers MA, Chen PY, Lin AYM, Seki Y (2008) Biological materials: Structure and mechanical properties. Prog Mater Sci 53: 1–206. doi:10.1016/j.pmatsci.2007.05.002
Nyman JS, Roy A, Shen XM, Acuna RL, Tyler JH, Wang XD (2006) The influence of water removal on the strength and toughness of cortical bone. J Biomech 39: 931–938. doi:10.1016/j.jbiomech.2005.01.012
Olszta MJ, Cheng XG, Jee SS, Kumar R, Kim YY, Kaufman MJ, Douglas EP, Gower LB (2007) Bone structure and formation: a new perspective. Mater Sci Eng R Rep 58: 77–116. doi:10.1016/j.mser.2007.05.001
Orgel JP, Wess TJ, Miller A (2000) The in situ conformation and axial location of the intermolecular cross-linked non-helical telopeptides of type I collagen. Struct Fold Des 8: 137–142
Orgel JPRO, Irving TC, Miller A, Wess TJ (2006) Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci USA 103: 9001–9005. doi:10.1073/pnas.0502718103
Orgel JPRO, Miller A, Irving TC, Fischetti RF, Hammersley AP, Wess TJ (2001) The in situ supermolecular structure of type I collagen. Structure 9: 1061–1069
Oyen ML (2008) The materials science of bone: lessons from nature for biomimetic materials synthesis. MRS Bull 33: 49–55
Parry DAD, Craig AS (1984) Growth and development of collagen fibrils in connective tissue. In: Ruggeri A, Motta PM (eds) Ultrastructure of the connective tissue matrix. Martinus Nijhoff, Boston, , pp 34–63
Perumal S, Antipova O, Orgel JPRO (2008) Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proc Natl Acad Sci USA 105: 2824–2829. doi:10.1073/pnas.0710588105
Posner AS (1969) Crystal chemistry of bone mineral. Physiol Rev 49: 760–792
Puxkandl R, Zizak I, Paris O, Keckes J, Tesch W, Bernstorff S, Purslow P, Fratzl P (2002) Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos Trans R Soc B Biol Sci 357: 191–197. doi:10.1098/rstb.2001.1033
Reid SA (1987) Micromorphological characterization of normal human-bone surfaces as a function of age. Scanning Micros 1: 579–597
Rho JY, Kuhn-Spearing L, Zioupos P (1998) Mechanical properties and the hierarchical structure of bone. Med Eng Phys 20: 92–102
Rubin MA, Jasiuk I, Taylor J, Rubin J, Ganey T, Apkarian RP (2003) TEM analysis of the nanostructure of normal and osteoporotic human trabecular bone. Bone 33: 270–282. doi:10.1016/S8756-3282(03)00194-7
Sasaki N, Enyo A (1995) Viscoelastic properties of bone as a function of water-content. J Biomech 28: 809–815
Shen H, Li H, Brinson LC (2008) Effect of microstructural configurations on the mechanical responses of porous titanium: a numerical design of experiment analysis for orthopedic applications. Mech Mater 40: 708–720. doi:10.1016/j.mechmat.008.03.009
Smith JW, Walmsley M (1959) Factors affecting the elasticity of bone. J Anat 93: 503–523
Traub W, Arad T, Weiner S (1989) 3-dimensional ordered distribution of crystals in turkey tendon collagen-fibers. Proc Natl Acad Sci USA 86: 9822–9826
Vincent JFV (1990) Structural biomaterials, revised edn. Princeton University, Princeton
Wang XD, Puram S (2004) The toughness of cortical bone and its relationship with age. Ann Biomed Eng 32: 123–135
Weiner S, Wagner HD (1998) The material bone: structure mechanical function relations. Ann Rev Mater Res 28: 271–298
Yamashita J, Furman BR, Rawls HR, Wang XD, Agrawal CM (2001) The use of dynamic mechanical analysis to assess the viscoelastic properties of human cortical bone. J Biomed Mater Res 58: 47–53
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, F., Stock, S.R., Haeffner, D.R. et al. A new model to simulate the elastic properties of mineralized collagen fibril. Biomech Model Mechanobiol 10, 147–160 (2011). https://doi.org/10.1007/s10237-010-0223-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-010-0223-9
Keywords
Profiles
- Stuart R. Stock View author profile