Abstract
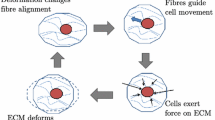
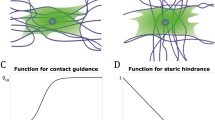
Microstructurally based models for bio-artificial tissues are needed to predict in vivo mechanical behavior and to validate assumptions for models of biologic tissues. We develop a microstructural model, based on on Zahalak et al. (2000) [Biophys 79(5):2369–2381], to describe matrix and tissue anisotropy observed in recent biaxial tests of fibroblast populated collagen vessels (FPCVs) with different cell orientations (Wagenseil et al. in Ann Biomed Eng 32(5):720–731 2004). The model includes pseudo-elastic cell behavior and pseudo-elastic, non-linear matrix behavior with recruitment of initially buckled collagen fibers. We obtained estimates of collagen matrix parameters from measurements of FPCVs treated with 2× 10−6 M Cytochalasin D and used these estimates to determine cell parameters in FPCVs activated with 5% fetal calf serum. The estimated stiffness of individual fibroblasts was 41–1,165 kPa. Parameter estimates for both cell and matrix were influenced by the non-linearity of the biaxial test data, making it difficult to obtain unique parameter values for some experiments. Additional microstructural measurements of the collagen matrix may help to more precisely determine the relative contributions of cells and matrix.
Similar content being viewed by others
References
Agoram B, Barocas VH (2001) Coupled macroscopic and microscopic scale modeling of fibrillar tissues and tissue equivalents. J Biomech Eng 123(4):362–369
Barocas VH, Tranquillo RT (1997) An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J Biomech Eng 119(2):137–145
Barocas VH, Moon AG, Tranquillo RT (1995) The fibroblast-populated collagen microsphere assay of cell traction force—part 2: measurement of the cell traction parameter. J Biomech Eng 117(2):161–170
Barocas VH, Girton TS, Tranquillo RT (1998) Engineered alignment in media equivalents: magnetic prealignment and mandrel compaction. J Biomech Eng 120(5):660–666
Bausch AR, Moller W, Sackmann E (1999) Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys J 76(1 Pt 1):573–579
Dobrin PB (1997). Chapter 3: physiology and pathophysiology of blood vessels. The basic science of vascular disease. Sidawy AN SB, DePalma RG. Futura Publishing, New York, pp 69–105
Eastwood M, McGrouther DA, Brown RA (1994) A culture force monitor for measurement of contraction forces generated in human dermal fibroblast cultures: evidence for cell-matrix mechanical signalling. Biochim Biophys Acta 1201(2):186–192
Fung YC (1993) Biomechanics: mechanical properties of living tissues. 2nd, Springer, Berlin Heidelberg New York
Fung YC, Liu SQ, Zhou JB (1993) Remodeling of the constitutive equation while a blood vessel remodels itself under stress. J Biomech Eng 115(4B):453–459
Girton TS, Oegema TR, Grassl ED, Isenberg BC, Tranquillo RT (2000) Mechanisms of stiffening and strengthening in media-equivalents fabricated using glycation. J Biomech Eng 122(3):216–223
Gleason RL, Humphrey JD (2004) A mixture model of arterial growth and remodeling in hypertension: Altered muscle tone and tissue turnover. J Vasc Res 41(4):352–363
Harris AK, Stopak D, Wild P (1981) Fibroblast traction as a mechanism for collagen morphogenesis. Nature 290(5803):249–251
Horowitz A, Lanir Y, Yin FC, Perl M, Sheinman I, Strumpf RK (1988) Structural three-dimensional constitutive law for the passive myocardium. J Biomech Eng 110(3):200–207
Kolodney MS, Wysolmerski RB (1992) Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol 117(1):73–82
Lanir Y (1983) Constitutive equations for fibrous connective tissues. J Biomech 16(1):1–12
Maksym GN, Fredberg JJ, Bates JH (1998) Force heterogeneity in a two-dimensional network model of lung tissue elasticity. J Appl Physiol 85(4): 1223–1229
Mardia KV, Jupp PE (2000) Directional statistics. 2nd edn. Wiley, New York
Marquez JP, Genin GM, Zahalak GI, Elson EL (2005a) Thin bio-artificial tissues in plane stress: the relationship between cell and tissue strain, and an improved constitutive model. Biophys J 88(2):765–777
Marquez JP, Genin GM, Zahalak GI, Elson EL (2005b) The relationship between cell and tissue strain in three-dimensional bio-artificial tissues. Biophys J 88(2):778–789
Sacks MS (2003) Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. J Biomech Eng 125(2):280–287
Taber LA (1991) On a nonlinear theory for muscle shells: part ii—application to the beating left ventricle. J Biomech Eng 113(1):63–71
Thoumine O, Ott A (1997) Time scale dependent viscoelastic and contractile regimes in fibroblasts probed by microplate manipulation. J Cell Sci 110(Pt 17):2109–2116
Tower TT, Neidert MR, Tranquillo RT (2002) Fiber alignment imaging during mechanical testing of soft tissues. Ann Biomed Eng 30(10):1221–1233
Wagenseil JE, Elson EL, Okamoto RJ (2004) Cell orientation influences the biaxial mechanical properties of fibroblast populated collagen vessels. Ann Biomed Eng 32(5):720–731
Wagenseil JE, Wakatsuki T, Okamoto RJ, Zahalak GI, Elson EL (2003) One-dimensional viscoelastic behavior of fibroblast populated collagen matrices. J Biomech Eng 125(5):719–725
Wakatsuki T, Kolodney MS, Zahalak GI, Elson EL (2000) Cell mechanics studied by a reconstituted model tissue. Biophys J 79:2353–2368
Yin FC, Chew PH, Zeger SL (1986) An approach to quantification of biaxial tissue stress–strain data. J Biomech 19(1):27–37
Zahalak GI, Wagenseil JE, Wakatsuki T, Elson EL (2000) A cell-based constitutive relation for bio-artificial tissues. Biophys J 79(5):2369–2381
Zar JH (1984) Biostatistical analysis. 2nd edn. Prentice-Hall, Englewood Cliffs
Zulliger MA, Fridez P, Hayashi K, Stergiopulos N (2004) A strain energy function for arteries accounting for wall composition and structure. J Biomech 37(7):989–1000
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagenseil, J.E., Okamoto, R.J. Modeling Cell and Matrix Anisotropy in Fibroblast Populated Collagen Vessels. Biomech Model Mechanobiol 6, 151–162 (2007). https://doi.org/10.1007/s10237-006-0019-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-006-0019-0