Abstract
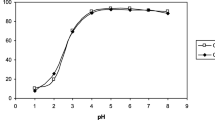
The objectives of this study were to investigate the adsorption of Cu-II and Zn-II on montmorillonite and bauxite and to discuss the usability of these adsorbents in environmental applications. The maximum adsorption was found for Cu (II) on bauxite with a Kf value of 4.179 L g−1; the maximum adsorbed capacity (Qo) calculated from the Langmuir model reached 9.115 mg g−1. Both raw materials have the potential to be effectively used to produce low-cost sorbents for copper and zinc removal from wastewater. They could be used as alternative adsorptive barriers and as amendments to soils at old mining areas, to prevent metal leaching into groundwater, or to isolate urban waste leachate.
Zusammenfassung
Die Ziele der vorliegenden Studie waren die Untersuchung der Adsorption von Cu-II und Zn-II-Ionen an Montmorillonit und Bauxit sowie die Diskussion der Verwendung dieser Adsorbentien für umweltrelevante Anwendungen. Die höchste Adsorption wurde mit einem KF-Wert von 4,179 L/g für Cu(II) an Bauxit gefunden. Die mittels Langmuir-Modell berechnete maximale Adsorptionskapazität (Qo) betrug 9,115 mg/g. Beide Rohstoffe können als preiswerte effektive Sorptionsmittel für Kupfer und Zink aus Abwässern genutzt zu werden. Sie können als alternative adsorptive Barriere und als Bodenzusatzstoffe in Altbergbaugebieten angewandt werden, um die Lösung von Metallen in das Grundwasser zu verhindern oder um Lösungsprodukte aus kommunalen Abfällen abzutrennen.
Resumen
Los objetivos de este estudio fueron investigar la adsorción de Cu-II y Zn-II sobre montmorillonita y bauxita y discutir la posibilidad del uso de estos adsorbentes en aplicaciones ambientales. La máxima adsorción se encontrada para Cu (II) sobre bauxita con un valor de Kf de 4,179 L g-1; la máxima capacidad de adsorción (Qo) calculada a partir del modelo de Langmuir fue 9,115 mg g-1. Ambos materiales tienen el potencial de ser efectivamente usados para producir sorbentes de bajo costo para la remoción de cobre y cinc desde aguas residuales. Estos sorbentes podrían ser usados como barraras adsortivas alternativas y como agregados para suelos en áreas mineras, para prevenir la lixiviación de metales dentro del agua subterránea o para aislar el lixiviado de residuos urbano.
抽象
文章旨在研究微晶高岭石和铝土矿的Cu(II)、Zn(II)吸附特征,讨论它们作为吸附剂的适用性。铝土矿对Cu(II)吸附性最强,Kf值为4.179 L g-1,通过Langmuir模型计算的最大吸附能力(Qo) 达9.115 mg g-1。两种原材料都具有成为去除废水铜和锌低成本吸附剂的潜力,可用作吸附屏障或老矿区土壤调理剂,用以阻止金属下渗进入地下水或隔离城市废物滤出液。





Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Atasoy AD (2008) Environmental problems in vertisol soils: the example of the Harran Plain. Fresen Environ Bull 17(7a):837–843
Atasoy AD, Yesilnacar MI, Şahin ÖM (2013) Removal of fluoride from contaminated ground water using raw and modified bauxite. Bull Environ Contam Toxicol 91:595–599
Bouhamed F, Elouear Z, Bouzid J, Ouddane B (2014) Batch sorption of Pb(II) ions from aqueous solutions using activated carbon prepared from date stones: equilibrium, kinetic, and thermodynamic studies. Desalin Water Treat 52:2261–2271
Bsoul AA, Zeatoun L, Abdelhay A, Chiha M (2014) Adsorption of copper ions from water by different types of natural seed materials. Desalin Water Treat 52:5876–5882
Eloussaief M, Jarraya I, Benzina M (2009) Adsorption of copper ions on two clays from Tunisia: pH and temperature effects. Appl Clay Sci 46(4):409–413
Gao S, Sun R, Wei Z, Zhao H, Li H, Hu F (2009) Size-dependent defluoridation properties of synthetic hydroxyapatite. J Fluor Chem 130:550–556
Gibert O, Cortina JL, Pablo J, Ayora C (2013) Performance of a field-scale permeable reactive barrier based on organic substrate and zero-valent iron for in situ remediation of acid mine drainage. Environ Sci Poll Res 20:7854–7862
Gove L, Cooke CM, Nicholson FA, Beck AJ (2001) Movement of water and heavy metals through sand and sandy loam amended wit biosolids under steady-state hydrological conditions. Bioresour Technol 78:171–179
Hedrich S, Johnson DB (2014) Remediation and selective recovery of metals from acidic mine waters using novel modular bioreactors. Environ Sci Technol 48:12206–12212
Huang K, Xiu Y, Zhu H (2014) Removal of heavy metal ions from aqueous solution by chemically modified mangosteen pericarp. Desalin Water Treat 52:7108–7116
Jennings SR, Dollhopf DJ (1995) Acid-base account effectiveness for determination of mine waste potential acidity. J Hazard Mater 41:161–175
Jung HS, Park JS, Lee YJ, Kim SK, Kong JY, Chun BS, Ryou JS (2013) Reduction of heavy metals and organic materials by atomized slag barrier in contaminated groundwater. KSCE J Civ Eng 17(7):1578–1586
Klauber C, Grafe M, Power G (2009) Review of bauxite residue “Re-use” options. CSIRO document DMR-3609, project ATF-06-03, management of bauxite residues. Department of Resources, Energy and Tourism (DRET), Australia
Komnitsas K, Bazdanis G, Bartzas G, Sahinkaya E, Zaharaki D (2013) Removal of heavy metals from leachates using organic/inorganic permeable reactive barriers. Desalin Water Treat 51:3052–3059
Kozyatnyk I, Haglund P, Lövgren L, Tysklind M, Gustafsson A, Törneman N (2014) Evaluation of barrier materials for removing pollutants from groundwater rich in natural organic matter. Water Sci Technol 70(1):32–39
Lombi E, Zhao F, Zhang G, Sun B, Fitz W, Zhang H, Mcgrath S (2002) In situ fixation of metals in soils using bauxite residue: chemical assessment. Environ Poll 118(3):435–443
Malarvizhi TS, Santhi T (2013) Adsorption of Zn (II) ions from aqueous solution on lignite-fired fly ash. Desalin Water Treat 51:6777–6788
Mermut AR, Padmanabham E, Eswaran H, Dasog GS (1996) Pedogenesis. In: Ahmad N, Mermut A (eds) Vertisols and technologies for their management development in soil science. Elsevier, Amsterdam, pp 43–61
Musso TB, Parolo ME, Pettinari G, Francisca FM (2014) Cu(II) and Zn(II) adsorption capacity of three different clay liner materials. J Environ Manag 146:50–58
Pavasant P, Apiratikul R, Sungkhum V, Suthiparinyanont P, Wattanachira S, Marhaba TF (2006) Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa Lentillifera. Bioresour Technol 97:2321–2329
Sdiri AT, Higashi T, Jamoussi F (2014) Adsorption of copper and zinc onto natural clay in single and binary systems. Int J Environ Sci Technol 11:1081–1092
Seliman AF, Lasheen YF, Youssief MAE, Abo-Aly MM, Shehata FA (2014) Removal of some radionuclides from contaminated solution using natural clay: bentonite. J Radioanal Nucl Ch 300:969–979
Shabalala AN, Ekolu SO, Diop S, Solomon F (2017) Pervious concrete reactive barrier for removal of heavy metals from acid mine drainage—column study. J Hazard Mater 323:641–653
Sheoran AS, Sheoran V (2006) Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Miner Eng 19:105–116
Si Y, Zhang J, Wang S, Zhang L, Zhou D (2006) Influence of organic amendment on the adsorption and leaching of ethametsulfuron-methyl in acidic soils in China. Geoderma 130:66–76
Sparks DL (1995) Environmental soil chemistry. Academic Press, San Diego
Younger PL, Banwart SA, Hedin RS (2002) Mine water: hydrology, pollution, remediation. Springer, New York
Zhaoa J, He MC (2014) Theoretical study of heavy metal Cd, Cu, Hg, and Ni(II) adsorption on the kaolinite (001) surface. Appl Surf Sci 317:718–723
Acknowledgements
This study was funded by the Scientific and Technological Research Council of Turkey (TUBITAK Project 110Y234) and the Scientific Research Projects Committee of Harran University (HÜBAK) under Grant 12163.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Atasoy, A.D., Bilgic, B. Adsorption of Copper and Zinc Ions from Aqueous Solutions Using Montmorillonite and Bauxite as Low-Cost Adsorbents. Mine Water Environ 37, 205–210 (2018). https://doi.org/10.1007/s10230-017-0464-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-017-0464-2