Abstract
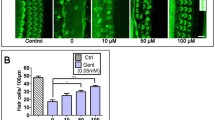
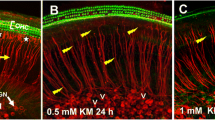
Gentamicin, one of the most widely used aminoglycoside antibiotics, is known to have toxic effects on the inner ear. Taken up by cochlear hair cells and spiral ganglion neurons (SGNs), gentamicin induces the accumulation of reactive oxygen species (ROS) and initiates apoptosis or programmed cell death, resulting in a permanent and irreversible hearing loss. Since the survival of SGNs is specially required for cochlear implant, new procedures that prevent SGN cell loss are crucial to the success of cochlear implantation. ROS modulates the activity of the mammalian target of rapamycin (mTOR) signaling pathway, which mediates apoptosis or autophagy in cells of different organs. However, whether mTOR signaling plays an essential role in the inner ear and whether it is involved in the ototoxic side effects of gentamicin remain unclear. In the present study, we found that gentamicin induced apoptosis and cell loss of SGNs in vivo and significantly decreased the density of SGN and outgrowth of neurites in cultured SGN explants. The phosphorylation levels of ribosomal S6 kinase and elongation factor 4E binding protein 1, two critical kinases in the mTOR complex 1 (mTORC1) signaling pathway, were modulated by gentamicin application in the cochlea. Meanwhile, rapamycin, a specific inhibitor of mTORC1, was co-applied with gentamicin to verify the role of mTOR signaling. We observed that the density of SGN and outgrowth of neurites were significantly increased by rapamycin treatment. Our finding suggests that mTORC1 is hyperactivated in the gentamicin-induced degeneration of SGNs, and rapamycin promoted SGN survival and outgrowth of neurites.






Similar content being viewed by others
References
Al-Ali H, Ding Y, Slepak T, Wu W, Sun Y, Martinez Y, Xu XM, Lemmon VP, Bixby JL (2017) The mTOR substrate S6 kinase 1 (S6K1) is a negative regulator of axon regeneration and a potential drug target for central nervous system injury. J Neurosci 37:7079–7095
Bae WY, Kim LS, Hur DY, Jeong SW, Kim JR (2008) Secondary apoptosis of spiral ganglion cells induced by aminoglycoside: Fas-Fas ligand signaling pathway. Laryngoscope 118:1659–1668
Bai X, Jiang Y (2010) Key factors in mTOR regulation. Cell Mol Life Sci 67:239–253
Chen Y, Zheng Y, Foster DA (2003) Phospholipase D confers rapamycin resistance in human breast cancer cells. Oncogene 22:3937–3942
Chen Y, Rodrik V, Foster DA (2005) Alternative phospholipase D/mTOR survival signal in human breast cancer cells. Oncogene 24:672–679
Chen L, Xiong S, Liu Y, Shang X (2012) Effect of different gentamicin dose on the plasticity of the ribbon synapses in cochlear inner hair cells of C57BL/6J mice. Mol Neurobiol 46:487–494
Ding D, Salvi R (2005) Review of cellular changes in the cochlea due to aminoglycoside antibiotics. Volta Rev 105:407–438
Ding D, Mcfadden SL, Browne RW, Salvi RJ (2003) Late dosing with ethacrynic acid can reduce gentamicin concentration in perilymph and protect cochlear hair cells. Hear Res 185:90–96
Ding D, Jiang H, Salvi RJ (2010) Mechanisms of rapid sensory hair-cell death following co-administration of gentamicin and ethacrynic acid. Hear Res 259:16–23
Ding D, Allman BL, Salvi R (2012) Review: ototoxic characteristics of platinum antitumor drugs. Anat Rec (Hoboken) 295:1851–1867
Fang B, Xiao H (2014) Rapamycin alleviates cisplatin-induced ototoxicity in vivo. Biochem Biophys Res Commun 448:443–447
Foster DA, Toschi A (2009) Targeting mTOR with rapamycin: one dose does not fit all. Cell Cycle 8:1026–1029
Ghasemnejad-Berenji M, Ghazi-Khansari M, Yazdani I, Saravi SSS, Nobakht M, Abdollahi A, Ansari JM, Ghasemnejad-Berenji H, Pashapour S, Dehpour AR (2017) Rapamycin protects testes against germ cell apoptosis and oxidative stress induced by testicular ischemia-reperfusion. Iran J Basic Med Sci 20:905–911
Hang J, Pan W, Chang A, Li S, Li C, Fu M, Tang J (2016) Synchronized progression of prestin expression and auditory brainstem response during postnatal development in rats. Neural Plast 2016:4545826
Hiel H, Erre JP, Aurousseau C, Bouali R, Dulon D, Aran JM (1993) Gentamicin uptake by cochlear hair cells precedes hearing impairment during chronic treatment. Audiology 32:78–87
Jeong SW, Kim LS, Hur D, Bae WY, Kim JR, Lee JH (2010) Gentamicin-induced spiral ganglion cell death: apoptosis mediated by ROS and the JNK signaling pathway. Acta Otolaryngol 130:670–678
Jiang M, Karasawa T, Steyger PS (2017) Aminoglycoside-induced cochleotoxicity: a review. Front Cell Neurosci 11:308
Kosek JC, Mazze RI, Cousins MJ (1974) Nephrotoxicity of gentamicin. Lab Invest 30:48–57
Kujawa SG, Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29:14077–14085
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149:274–293
Leitmeyer K, Glutz A, Radojevic V, Setz C, Huerzeler N, Bumann H, Bodmer D, Brand Y (2015) Inhibition of mTOR by rapamycin results in auditory hair cell damage and decreased spiral ganglion neuron outgrowth and neurite formation in vitro. Biomed Res Int 2015:925890
Li M, Zhao L, Liu J, Liu A, Jia C, Ma D, Jiang Y, Bai X (2010) Multi-mechanisms are involved in reactive oxygen species regulation of mTORC1 signaling. Cell Signal 22:1469–1476
Li Y, Ding D, Jiang H, Fu Y, Salvi R (2011) Co-administration of cisplatin and furosemide causes rapid and massive loss of cochlear hair cells in mice. Neurotox Res 20:307–319
Li C, Chen S, Yu Y, Zhou C, Wang Y, Le K, Li D, Shao W, Lu L, You Y, Peng J, Huang H, Liu P, Shen X (2014) BIG1, a brefeldin A-inhibited guanine nucleotide-exchange factor, is required for GABA-gated Cl(−) influx through regulation of GABAA receptor trafficking. Mol Neurobiol 49:808–819
Liu K, Jiang X, Shi C, Shi L, Yang B, Xu Y, Yang W, Yang S (2013) Cochlear inner hair cell ribbon synapse is the primary target of ototoxic aminoglycoside stimuli. Mol Neurobiol 48:647–654
Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA (2010) Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci 30:1166–1175
Mccormick F (2004) Cancer: survival pathways meet their end. Nature 428:267–269
Pan Y, Nishida Y, Wang M, Verdin E (2012) Metabolic regulation, mitochondria and the life-prolonging effect of rapamycin: a mini-review. Gerontology 58:524–530
Park YS, Park JH, Ko J, Shin IC, Koh HC (2017) mTOR inhibition by rapamycin protects against deltamethrin-induced apoptosis in PC12 cells. Environ Toxicol 32:109–121
Ruan Q, Ao H, He J, Chen Z, Yu Z, Zhang R, Wang J, Yin S (2014) Topographic and quantitative evaluation of gentamicin-induced damage to peripheral innervation of mouse cochleae. Neurotoxicology 40:86–96
Saqcena M, Patel D, Menon D, Mukhopadhyay S, Foster DA (2015) Apoptotic effects of high-dose rapamycin occur in S-phase of the cell cycle. Cell Cycle 14:2285–2292
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22:159–168
Selimoglu E (2007) Aminoglycoside-induced ototoxicity. Curr Pharm Des 13:119–126
Toschi A, Lee E, Gadir N, Ohh M, Foster DA (2008) Differential dependence of hypoxia-inducible factors 1α and 2α on mTORC1 and mTORC2. J Biol Chem 283:34495–34499
Warchol ME (2010) Cellular mechanisms of aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg 18:454–458
Wu WJ, Sha SH, Mclaren JD, Kawamoto K, Raphael Y, Schacht J (2001) Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res 158:165–178
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124:471–484
Yu Q, Chang Q, Liu X, Wang Y, Li H, Gong S, Ye K, Lin X (2013) Protection of spiral ganglion neurons from degeneration using small-molecule TrkB receptor agonists. J Neurosci 33:13042–13052
Zhao N, Tai X, Zhai L, Shi L, Chen D, Yang B, Ji F, Hou K, Yang S, Gong S, Liu K (2017) Unitary ototoxic gentamicin exposure may not disrupt the function of cochlear outer hair cells in mice. Acta Otolaryngol 137:842–849
Acknowledgments
This work was supported by the 973 Program of China (grant number 2014CB943002), the National Natural Science Foundation of China (grant number 31500841), the Guangdong Natural Science Foundation (grant number 2017A030313178), the Program for Changjiang Scholars and Innovative Research Team in University (IRT16R37), and Medical Scientific Research Foundation of Guangdong Province (grant number A2015445).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, S., Xu, N., Chen, P. et al. Rapamycin Protects Spiral Ganglion Neurons from Gentamicin-Induced Degeneration In Vitro. JARO 20, 475–487 (2019). https://doi.org/10.1007/s10162-019-00717-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-019-00717-3