Abstract
Background
No studies have been published on the correlation between lactic dehydrogenase-to-albumin ratio (LAR) and poor prognosis of acute kidney injury (AKI) patients, warranting further research. This analysis sought to investigate the prognostic implication of LAR in critically ill patients with AKI.
Methods
The present study enrolled 11,046 and 5180 adults with AKI from the Medical Information Mart for Intensive Care III (MIMIC III) and MIMIC IV, respectively. Data from MIMIC IV were identified as the training cohort, and those from MIMIC III were identified as the validation cohort. We applied multivariate regression analysis to identify the link between LAR and all-cause mortality. Restricted cubic spline (RCS) was conducted to figure out the correlation between LAR and in-hospital mortality. Furthermore, we carried out stratification analyses to examine if the effects of LAR on in-hospital mortality were consistent across various subclasses.
Results
The level of LAR was remarkably higher in the in-hospital non-survivor group (p < 0.001). Furthermore, the increased LAR group presented a remarkably higher rate of in-hospital mortality at AKI stages 1, 2, and 3 compared with the decreased LAR group (all p < 0.001). Multivariate regression analyses exhibited the independent prognostic significance of LAR for all-cause mortality (all p < 0.001). MIMIC III observed concordant results. RCS indicated a non-linear correlation between LAR and in-hospital death (P for non-linearity < 0.001). The relationship between LAR and in-hospital mortality was still significant in patients with various subclasses.
Conclusions
Elevated LAR at admission is a prognostic risk factor for critically ill patients with AKI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is a prevalent comorbidity in intensive care unit (ICU) patients, with an incidence of up to 50%, correlated with prolonged hospital stays, increased mortality, and excess healthcare costs [1, 2]. Thus, identifying high-risk AKI individuals is essential for timely and effective interventions to improve patient outcomes. In recent years, new AKI prognostic predictors have been documented [3,4,5]. Unfortunately, applying these biomarkers in clinical practice still faces significant limitations.
Lactate dehydrogenase (LDH) is a key enzyme of glycolysis that has associated with worse outcomes in patients with severe acute diseases, including sepsis [6], severe acute pancreatitis [7], and severe COVID-19 [8]. Besides, studies have revealed an association between LDH and the progression of diabetic kidney disease [9], and mortality in incident hemodialysis patients [10]. Serum albumin, a commonly used marker of nutritional status, has been demonstrated to be correlated with the development of AKI and death after AKI [11, 12]. An increasing body of evidence suggests that the LDH-to-serum albumin ratio (LAR), the combination of them, is strongly related to poor outcomes in patients with cancer [13, 14] and infectious diseases [15, 16]. Nevertheless, no studies have hitherto assessed the relationship between LAR and AKI patient mortality. Accordingly, this paper sought to examine the relationship between LAR upon admission and AKI patient mortality in the ICU using data from two public databases.
Materials and methods
Study design
Data were collected from two large open critical care databases, the Medical Information Mart for Intensive Care III (MIMIC III) and MIMIC IV. MIMIC IV, which includes ICU patients treated at Beth Israel Deaconess Medical Center from 2008 to 2019, was employed to examine the relationship between LAR at admission and mortality in ICU patients with AKI [17]. MIMIC III, which contains data from 2001 to 2012, was carried out to validate the results [17]. The authors were entitled to extract data from the two databases after completion of the course "Protecting Human Research Participants".
Population selection criteria
This analysis enrolled adult patients (18 years or older) diagnosed with AKI. We excluded patients with missing data for serum albumin or LDH, multiple ICU admissions, having stayed in the ICU less than 2 days, and duplicated records in both databases. In the case of multiple ICU admissions, only the first admission was selected.
Data extraction and definitions
Variables extracted contained demographic data (age and sex), vital signs (heart rate and blood pressure), comorbidities (heart failure, liver cirrhosis, malignancy, sepsis, etc.), laboratory parameter [white blood cell (WBC), hemoglobin, etc.], scoring systems [simplified acute physiology score (SAPS) II, sequential organ failure assessment (SOFA)], and intervention measures [continuous renal replacement therapy (CRRT), vasopressors, and mechanical ventilation]. The laboratory indicators were taken from the first measurement recorded after admission. AKI was defined and staged according to the "Kidney Disease Improving Global Outcomes" criteria [18]. The LAR was calculated by initial serum LDH (U/L) /serum albumin (g/L). The in-hospital mortality was the primary outcome, while ICU mortality, 30-day, 90-day, and 365-day all-cause mortality from the date of admission were regarded as secondary outcomes.
Statistical analysis
Continuous variables were summarized as medians with an interquartile range, and differences were examined using the Mann–Whitney U test, since the variables exhibited a skewed distribution. Categorical data were described as frequencies with proportions, and the differences between groups were identified by the Chi-square test. We generated a receiver-operating characteristic (ROC) curve to identify the optimal cut-off point of LAR for predicting in-hospital mortality. The cut-off value was used to split patients into two groups. 365-Day cumulative survival between the two groups was compared by the Kaplan–Meier (KM) curve with the log-rank test. We employed multivariate logistic and Cox proportional hazards regression analysis to identify the link between LAR and all-cause mortality. LAR was examined as both a continuous and a categorical variable. Factors related to in-hospital death during univariate analyses in the MIMIC IV database were applied to establish a multivariable regression model. These covariates included age, systolic blood pressure, heart rate, hypertension, coronary heart disease, chronic kidney disease, liver cirrhosis, malignancy, sepsis, WBC, hemoglobin, alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, sodium, potassium, anion gap, bicarbonate, SAPS II, SOFA score, CRRT, vasopressors, and ventilation. A restricted cubic spline with three knots was established to figure out the correlation between LAR and in-hospital mortality. Further analysis was conducted after stratification according to AKI stages, age, gender, comorbidities, and intervention measures. We performed all statistical analysis by R software (version 3.6.3). A P value < 0.05 was statistically significant.
Results
Basic characteristics
After screening patients based on the inclusion and exclusion criteria, 5180 and 11,046 patients were included from the MIMIC III and MIMIC IV databases, respectively (Supplementary Fig. 1). A comparison of the characteristics of patients from the two databases at baseline is presented in Supplement Table 1. The two databases were heterogeneous to some extent. The patient characteristics in the survival and mortality groups are presented in Table 1. Overall, LDH and LAR levels were remarkably higher in the in-hospital non-survivor group, whereas serum albumin levels were lower (p < 0.001). Compared with survivors in the MIMIC IV database, patients who died during hospitalization had higher age, SAPS II, SOFA score, faster heart rate, lower SBP, higher prevalence of CKD, liver cirrhosis, malignancy, sepsis, lower proportions of hypertension, and CHD, and were more likely to receive CRRT, mechanical ventilation, and used more vasopressors. In terms of laboratory indices, patients with in-hospital death exhibited higher levels of WBC, BUN, SCr, potassium, anion gap, ALT, and AST, but lower levels of bicarbonate, sodium, and hemoglobin. Analysis of patients from the MIMIC III database showed that the levels of heart rate, WBC, BUN, SCr, potassium, anion gap, ALT, AST, SAPS II, and SOFA score were significantly higher, but SBP, DBP, hemoglobin, sodium, bicarbonate were lower in the in-hospital death group when compared with the survival group. Besides, mechanical ventilation, vasopressors, and CRRT were more common in patients who died during hospitalization. Moreover, remarkable differences in the history of hypertension, diabetes, CHD, heart failure, liver cirrhosis, malignancy, and sepsis were also observed between both groups.
Association between LDH and outcomes
Patients were categorized into low (≤ 10.6) and high (> 10.6) LAR groups based on the best cut-off score determined by ROC curve analysis (Supplementary Fig. 2). MIMIC IV database showed that the incidence of in-hospital, ICU, 30-day, 90-day, and 365-day mortality increased significantly in patients with increased LAR compared with those with decreased LAR, in line with results in the MIMIC III database (Supplementary Table 2). When stratified by the AKI stage, in-hospital mortality rates of the increased LAR group at stages 1, 2, and 3 were remarkably higher than the decreased LAR group in both databases (all p < 0.001; Fig. 1A, B).
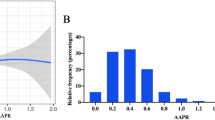
The predictive significance of LAR for all-cause mortality was verified by multivariate regression analyses. During analysis of the MIMIC IV database showed that, even after controlling confounding factors, a one-unit increase of LAR was strongly correlated with increased risk of in-hospital, ICU, 30-day, 90-day, and 365-day mortality (all p < 0.001, Table 2). The results also exhibited that high LAR was an important predictor of all-cause mortality [OR (95% CI) for in-hospital mortality: 2.14 (1.91–2.40), p < 0.001; OR (95% CI) for ICU mortality: 2.21 (1.95–2.50), p < 0.001; HR (95% CI) for 30-day mortality: 1.91 (1.75–2.08), p < 0.001; HR (95% CI) for 90-day mortality: 1.91 (1.74–2.08), p < 0.001; HR (95% CI) for 365-day mortality: 1.86 (1.71–2.03), p < 0.001, Table 2]. Similar outcomes were noted during the analysis of the MIMIC III database for the impact of LAR on clinical outcomes. Multivariate regression analysis revealed the independent prognostic significance of LAR for all-cause mortality, whether considered LAR as a nominal or continuous variable (all p < 0.001, Table 2). KM curves for the 365-day cumulative survival rate based on the best cut-off score of LAR in both databases are illustrated in Fig. 2. KM curves for 365-day death in the two databases presented a significant statistical difference between the increased and decreased LAR group (Fig. 2A, B; both log-rank P < 0.001). A non-linear correlation between LAR and in-hospital mortality was obtained in Fig. 2C (P for non-linearity < 0.001). The probability of in-hospital mortality increased rapidly with the LAR level up to 18.05 (OR (95% CI):1.09 (1.08–1.10), p < 0.001, Table 3). When the level of LAR is more than 18.05, the probability of in-hospital death increased relatively slowly.
Kaplan–Meier curves and restricted cubic spline analysis. Kaplan–Meier curves for 365-day accumulative survival rates stratified by high and low LAR in MIMIC IV (A) and MIMIC III (B) databases. The association between LAR and in-hospital death in MIMIC IV using restricted cubic spline analysis (C). A non-linear association between LAR and in-hospital mortality was observed after adjusting for age, SBP, heart rate, hypertension, CHD, CKD, liver cirrhosis, malignancy, sepsis, WBC, hemoglobin, BUN, sodium, potassium, anion gap, bicarbonate, ALT, AST, SAPS II, SOFA score, CRRT, vasopressors, and ventilation. LAR, lactic dehydrogenase-to-albumin ratio; MIMIC, Medical Information Mart for Intensive Care
Subgroup analyses
Subgroup analyses were carried out to assess the risk stratification performance of LAR for in-hospital mortality using the MIMIC IV database (Fig. 3). The high LAR was consistently associated with increased in-hospital death in different subgroups, including AKI stage 1 to 3, age ≤ 65 or > 65 years, female or male, with or without hypertension, diabetes, CHD, heart failure, CKD, liver cirrhosis, malignancy, sepsis, CRRT, vasopressors, and mechanical ventilation. Notably, the predictive value of LAR was more pronounced in patients without CKD (Pinteraction = 0.035) and patients with malignancy (Pinteraction = 0.033), and patients who did not undergo CRRT (Pinteraction = 0.009) and mechanical ventilation (Pinteraction < 0.001).
Discussion
The principal finding was that LAR at admission was remarkably correlated with an increased risk of all-cause mortality in critically ill patients with AKI after multiple covariates’ adjustment. Most importantly, these correlations remained significant after stratifying according to AKI stages. The correlation of LAR with all-cause mortality was further confirmed using data from the MIMIC III database.
Prior studies have detected that LDH is strongly related to poor prognoses in various clinical settings, including infectious diseases [6, 8], cancer [19, 20], cardiovascular disease [21], cardiac surgery [22], and hypoxic hepatitis [23]. Recent work has established the relationship between LDH and long-term mortality in incident hemodialysis patients [10]. Besides, LDH and hypoalbuminemia have been demonstrated to be crucial markers for predicting prognosis in critically ill patients [24, 25]. Moreover, the relationship between hypoalbuminemia and unfavorable endpoints in patients with AKI in multiple clinical scenarios has been established [26, 27]. To our knowledge, no research has focused on the predictive implications of LAR in ICU patients with AKI. Our analysis substantiated that LAR is a vital predictor of all-cause mortality in ill critical patients with AKI. The prognostic significance of LAR was further verified in the external validation, indicating the reliability of our results. Besides, the correlation between LAR and in-hospital mortality was still observed after stratification according to the AKI stages, which showed that the prognostic implication of LAR was not affected by the severity of kidney injury.
LDH is widely acknowledged as a prognostic risk factor for multiple diseases. There is a rich literature substantiating that hypoalbuminemia is correlated with poor prognosis in various diseases, such as cancer [28, 29], cardiovascular diseases [30], heart failure [31], and sepsis [32]. The findings of subgroup analysis suggested that the predictive significance of LAR seemed not to be affected by disease types. Indeed, it is well established that albumin is susceptible to changes at age [33]. Subgroup analysis by age showed no differences in the prognostic ability of LAR. There is a possibility that LAR can reduce these uncertainty biases. LAR, which combines LDH and albumin, may yield a better predictive value than the individual indicators. However, it remains unclear why LAR exhibited more significant predictive value in patients that did not receive CRRT and mechanical ventilation. We speculate that LAR might serve as a useful biomarker for relatively low-risk AKI patients.
At present, the specific mechanism regarding the link of LAR with all-cause mortality remains uncertain. The following are some speculations. It is widely thought that albumin possesses anti-inflammatory properties [34] and exhibits significant value as an inflammation indicator in infectious diseases [35]. LDH has also been reported as an inflammation marker of pulmonary diseases [36, 37]. These studies showed that albumin and LDH were associated with inflammation, which is a vital pathogenic mechanism in the development and progression of AKI [38, 39]. Hypoperfusion is another significant cause of AKI progression [38, 39]. Hypoalbuminemia could cause leakage of intravascular fluid which results in a further decrease of blood volume and exacerbate renal hypoperfusion [26, 39]. LDH is a crucial enzyme in anaerobic glycolysis that can catalyze pyruvate to lactate [40]. Hypoperfusion is often accompanied by tissue ischemia and hypoxia, which contribute to the rapid accumulation of lactate. Legouis et al. revealed that impaired lactate clearance is closely correlated with the mortality of AKI patients [41].
Although the present study used data from two large critical care databases, some limitations remain. First, LAR was only evaluated at admission; thus, it is unclear whether dynamic changes in LAR can predict prognosis. Second, despite controlling several confounding variables during multivariate analysis, selection bias may affect our findings' robustness to a certain extent. Third, finally, the present study did not explore the mechanism behind the relationship between all-cause mortality and elevated LAR.
Conclusion
The LAR upon admission is a crucial predictor for all-cause mortality of AKI patients in the ICU.
References
Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational aki-epi study. Intensive Care Med. 2015;41:1411–23.
Silver SA, Long J, Zheng Y, Chertow GM. Cost of acute kidney injury in hospitalized patients. J Hosp Med. 2017;12:70–6.
Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med. 2017;55:1074–89.
Oh DJ. A long journey for acute kidney injury biomarkers. Ren Fail. 2020;42:154–65.
McMahon BA, Galligan M, Redahan L, Martin T, Meaney E, Cotter EJ, et al. Biomarker predictors of adverse acute kidney injury outcomes in critically ill patients: the Dublin acute biomarker group evaluation study. Am J Nephrol. 2019;50:19–28.
Lu J, Wei Z, Jiang H, Cheng L, Chen Q, Chen M, et al. Lactate dehydrogenase is associated with 28-day mortality in patients with sepsis: a retrospective observational study. J Surg Res. 2018;228:314–21.
Kaya E, Dervisoglu A, Polat C. Evaluation of diagnostic findings and scoring systems in outcome prediction in acute pancreatitis. World J Gastroenterol. 2007;13:3090–4.
Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill covid-19 patients: a review. Allergy. 2021;76:428–55.
Al-Rubeaan K, Siddiqui K, Alghonaim M, Youssef AM, AlNaqeb D. The Saudi diabetic kidney disease study (Saudi-dkd): Clinical characteristics and biochemical parameters. Ann Saudi Med. 2018;38:46–56.
Ryu SY, Kleine CE, Hsiung JT, Park C, Rhee CM, Moradi H, et al. Association of lactate dehydrogenase with mortality in incident hemodialysis patients. Nephrol Dial Transplant. 2021;36:704–12.
Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med. 2010;36:1657–65.
Hansrivijit P, Yarlagadda K, Cheungpasitporn W, Thongprayoon C, Ghahramani N. Hypoalbuminemia is associated with increased risk of acute kidney injury in hospitalized patients: a meta-analysis. J Crit Care. 2021;61:96–102.
Feng JF, Wang L, Yang X, Jiang YH. Prognostic value of lactate dehydrogenase-to-albumin ratio (lar) in patients with resectable esophageal squamous cell carcinoma. Cancer Manag Res. 2019;11:7243–51.
Peng RR, Liang ZG, Chen KH, Li L, Qu S, Zhu XD. Nomogram based on lactate dehydrogenase-to-albumin ratio (lar) and platelet-to-lymphocyte ratio (plr) for predicting survival in nasopharyngeal carcinoma. J Inflamm Res. 2021;14:4019–33.
Yan D, Huang Q, Dai C, Ren W, Chen S. Lactic dehydrogenase-to-albumin ratio is associated with the risk of stroke-associated pneumonia in patients with acute ischemic stroke. Front Nutr. 2021;8: 743216.
Lee BK, Ryu S, Oh SK, Ahn HJ, Jeon SY, Jeong WJ, et al. Lactate dehydrogenase-to-albumin ratio as a prognostic factor in lower respiratory tract infection patients. Am J Emerg Med. 2022;52:54–8.
Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. Mimic-iv, a freely accessible electronic health record dataset. Sci Data. 2023;10:1.
Ad-hoc working group of E, Fliser D, Laville M, Covic A, Fouque D, Vanholder R, et al. A european renal best practice (erbp) position statement on the kidney disease improving global outcomes (kdigo) clinical practice guidelines on acute kidney injury: Part 1: Definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27:4263–72.
Pelizzari G, Basile D, Zago S, Lisanti C, Bartoletti M, Bortot L, et al. Lactate dehydrogenase (ldh) response to first-line treatment predicts survival in metastatic breast cancer: first clues for a cost-effective and dynamic biomarker. Cancers (Basel). 2019;11:1243.
Forkasiewicz A, Dorociak M, Stach K, Szelachowski P, Tabola R, Augoff K. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell Mol Biol Lett. 2020;25:35.
Liao YT, Chen CJ, Li WF, Hsu LI, Tsai LY, Huang YL, et al. Elevated lactate dehydrogenase activity and increased cardiovascular mortality in the arsenic-endemic areas of southwestern taiwan. Toxicol Appl Pharmacol. 2012;262:232–7.
Zeng Y, Zhao Y, Dai S, Liu Y, Zhang R, Yan H, et al. Impact of lactate dehydrogenase on prognosis of patients undergoing cardiac surgery. BMC Cardiovasc Disord. 2022;22:404.
Aboelsoud MM, Javaid AI, Al-Qadi MO, Lewis JH. Hypoxic hepatitis - its biochemical profile, causes and risk factors of mortality in critically-ill patients: a cohort study of 565 patients. J Crit Care. 2017;41:9–15.
Su D, Li J, Ren J, Gao Y, Li R, Jin X, et al. The relationship between serum lactate dehydrogenase level and mortality in critically ill patients. Biomark Med. 2021;15:551–9.
Padkins M, Breen T, Anavekar N, Barsness G, Kashani K, Jentzer JC. Association between albumin level and mortality among cardiac intensive care unit patients. J Intensive Care Med. 2021;36:1475–82.
Lv J, Wang H, Sun B, Gao Y, Zhang Z, Pei H. Serum albumin before crrt was associated with the 28- and 90-day mortality of critically ill patients with acute kidney injury and treated with continuous renal replacement therapy. Front Nutr. 2021;8: 717918.
Wiedermann CJ, Wiedermann W, Joannidis M. Causal relationship between hypoalbuminemia and acute kidney injury. World J Nephrol. 2017;6:176–87.
Haskins IN, Baginsky M, Amdur RL, Agarwal S. Preoperative hypoalbuminemia is associated with worse outcomes in colon cancer patients. Clin Nutr. 2017;36:1333–8.
Yoshikawa N, Yoshihara M, Tamauchi S, Ikeda Y, Yokoi A, Kajiyama H. Hypoalbuminemia for the prediction of survival in patients with stage ivb cervical cancer. PLoS ONE. 2022;17: e0273876.
Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12.
Bonilla-Palomas JL, Gamez-Lopez AL, Moreno-Conde M, Lopez-Ibanez MC, Anguita-Sanchez M, de la Sacristana AG, et al. Hypoalbuminemia in acute heart failure patients: Causes and its impact on hospital and long-term mortality. J Card Fail. 2014;20:350–8.
Furukawa M, Kinoshita K, Yamaguchi J, Hori S, Sakurai A. Sepsis patients with complication of hypoglycemia and hypoalbuminemia are an early and easy identification of high mortality risk. Intern Emerg Med. 2019;14:539–48.
Salive ME, Cornoni-Huntley J, Phillips CL, Guralnik JM, Cohen HJ, Ostfeld AM, et al. Serum albumin in older persons: Relationship with age and health status. J Clin Epidemiol. 1992;45:213–21.
Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17:432–7.
Wiedermann CJ. Hypoalbuminemia as surrogate and culprit of infections. Int J Mol Sci. 2021;22:4496.
Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736–42.
Faruqi S, Wilmot R, Wright C, Morice AH. Serum ldh in chronic cough: a potential marker of airway inflammation. Clin Respir J. 2012;6:81–7.
Wu YL, Li HF, Chen HH, Lin H. Micrornas as biomarkers and therapeutic targets in inflammation- and ischemia-reperfusion-related acute renal injury. Int J Mol Sci. 2020;21:6738.
Peerapornratana S, Manrique-Caballero CL, Gomez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083–99.
Cobben NA, Drent M, Schols AM, Lamers RJ, Wouters EF, Van Dieijen-Visser MP. Serum lactate dehydrogenase and its isoenzyme pattern in ex-coalminers. Respir Med. 1997;91:616–23.
Legouis D, Ricksten SE, Faivre A, Verissimo T, Gariani K, Verney C, et al. Altered proximal tubular cell glucose metabolism during acute kidney injury is associated with mortality. Nat Metab. 2020;2:732–43.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have declared no competing interest.
Ethical approval
The Institutional Review Boards of the Massachusetts Institute of Technology exempted the MIMIC III/IV database from ethics review, given that patient information was hidden to protect privacy.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Deng, Y., Li, X., Lai, Q. et al. Prognostic implication of lactic dehydrogenase-to-albumin ratio in critically ill patients with acute kidney injury. Clin Exp Nephrol 27, 349–357 (2023). https://doi.org/10.1007/s10157-023-02321-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-023-02321-5