Abstract
Background
Tolvaptan (TLV) is reported to improve diuretic effects in patients with chronic kidney disease (CKD) when furosemide (FUR) is not sufficiently effective. However, it is not clear whether TLV addition is effective for advanced CKD patients with heart failure.
Methods
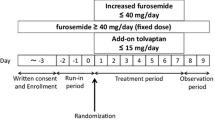
An open-label, parallel-group randomized trial was performed. The subjects were 33 patients with CKD stage G3–G5 who had fluid overload despite taking 20–100 mg/day FUR. They were divided into two groups: a group administered 15 mg/day TLV plus their original FUR dose for 7 days (TLV group), and a group administered 120–200 mg/day FUR (i.e., 100 mg/day over their previous dose) for 7 days (FUR group).
Results
The mean change in urine volume was significantly higher in the TLV group compared to the FUR group (637 ml vs 119 ml; p < 0.05). The difference was greater when the urine osmolality before treatment was high. Serum creatinine was increased only in the FUR group. The incidence of worsening renal function (WRF) was significantly lower in the TLV group (18.8% vs 58.8%; p < 0.05). Serum sodium decreased significantly in the FUR group, but did not change in the TLV group.
Conclusions
In patients with advanced CKD with fluid overload, the addition of TLV achieved a significantly higher urine volume with less adverse effects on renal function compared with increasing the dose of FUR. The efficacy and safety of TLV were higher in patients who had higher urine osmolality and lower serum sodium before treatment.
Clinical trial registration
UMIN000014763.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Loop diuretics such as furosemide (FUR) have been used to treat fluid overload in patients with heart failure [1]. However, patients with chronic kidney disease (CKD) often do not respond well to loop diuretics [2]. The lower the renal function, the worse is the response to loop diuretics, and increasing the dose of diuretics to improve the response often causes side effects such as deterioration of renal function and electrolyte imbalance [3]. In some cases, dialysis may be required due to inadequate therapeutic effect even after increasing the dose of diuretics.
Tolvaptan (TLV) binds to vasopressin V2 receptors and inhibits water reabsorption in the renal collecting ducts [4]. When used in combination with loop diuretics, TLV has been reported to improve fluid overload [5,6,7]. Moreover, in recent years, the combination of TLV with loop diuretics has been reported to be more effective than loop diuretics alone in CKD patients [8,9,10,11,12].
However, many of these reports targeted patients with mild-to-moderate CKD. TLV is thought to be less effective as renal function declines [13], and it has not been clarified how effective TLV is in patients with markedly reduced renal function and high diuretic resistance. In our previous retrospective study, we found that TLV was effective in patients with advanced CKD [12], but few studies have prospectively examined the efficacy of TLV in patients with advanced CKD. Therefore, this prospective randomized controlled study was designed to compare the effects of TLV addition versus increased FUR dose in advanced CKD patients. In addition, by examining the factors correlated with those effects, we will examine the indications for future use of tolvaptan.
Methods
This study was a multicenter, open-label, randomized controlled trial. We enrolled CKD patients in stages G3–G5 with heart failure who were treated at either Yokohama City University Medical Center or Yokohama City University Hospital between July 2015 and July 2020 and who had at least one sign of fluid excess (pleural effusion, ascites, lower leg edema, eyelid edema, pulmonary congestion, or jugular vein distension) despite taking 20–100 mg/day FUR. CKD was diagnosed based on the guidelines of the Japanese Society of Nephrology [14]. Heart failure was diagnosed based on the guidelines of the Japanese Circulation Society [15]. Exclusion criteria included anuria, dialysis, hypernatremia, history of TLV use, hypersensitivity to tolvaptan, and difficulty drinking freely.
The patients were observed for 3 days without changing their FUR dosage. The patients were then randomized into two groups by minimization method using urine volume and serum creatinine levels during the observation period as adjustment factors. The TLV group was treated for 7 days with continued FUR at the baseline dosage plus 15 mg/day TLV. The FUR group was administered 120–200 mg/day FUR (i.e., 100 mg/day over their previous dose). Treatment was inpatient and salt intake was limited to 5 g/day. There was no limit to the amount of water consumed. Urine volume, water intake, and body weight were measured daily, and blood and urine were collected on days 1, 2, 5, and 8 of treatment.
The primary end point of this study was the change in mean urine volume compared to baseline values. Secondary endpoints were change in serum creatinine, incidence of worsening renal function (WRF) (defined as an increase in serum creatinine of 0.3 mg/dl or more from baseline), change in body weight, and change in serum sodium. For urine volume, the mean value during the observation period was defined as the baseline. For other items, the value immediately before the start of treatment was defined as the baseline.
Based on previous reports [12, 16], the change in urine volume (the primary endpoint) was estimated to be + 500 ml/day (SD = 450 ml/day) in the TLV group and + 100 ml/day (SD = 450 ml/day) in the FUR group. We calculated the sample size based on a t test assuming equal variances at a two-sided significance level of 5% and a power of 80%, and calculated that 20 patients per group were needed. To account for potential dropouts, the target number of cases was set at 25 cases each, or a total of 50 cases in the two groups.
The full analysis set (FAS) was analyzed. Because the number of missing values was small and missing completely at random, we performed a complete case analysis. Continuous variables were reported as mean ± SD. Continuous variables were compared using the Student’s t test for normal distribution and the Wilcoxon rank sum test when the distribution assumption was not met. Linear regression analysis was used to elucidate the relationship between clinical parameters and endpoints. The incidence of WRF was compared by χ2 test. Patients were divided into two subgroups by median baseline urine osmolality, and changes in urine volume within each group were compared between the two drugs. Multiple regression analysis was used to investigate the effect of drug selection on changes in urine volume. Significance was defined at p < 0.05 using two-sided tests. Data analysis was performed using JMP Pro 15.0 (2019; SAS, Cary, NC).
The study protocol was approved by the Ethics Committee of Yokohama City University (D1405025) and was registered at the University Hospital Medical Information Network clinical trial registry (ID: UMIN000014763).
Results
In all, 35 patients were randomized. One patient met the exclusion criteria and did not receive study treatment. 1 patient was found not to meet the diagnostic criteria for heart failure and was excluded. A total of 33 patients were thus treated and used for analysis. The baseline characteristics of the patients are shown in Table 1. The patients were predominantly male and ranged in age from 44 to 84 years. eGFR was 13.7 and 13.8. The mean dose of FUR prior to randomization was 60 ± 25 mg/day. With the exception of the baseline dose of FUR, the baseline parameters were not significantly different between the two groups.
Prior to day 7, three patients (19%) in the TLV group and three (18%) in the FUR group discontinued the treatment protocol. Reasons for discontinuation were early completion of treatment due to the resolution of fluid overload (one patient in each group), dry mouth and inadequate efficacy in the TLV group, and blood pressure decrease and patient request in the FUR group.
Primary end point
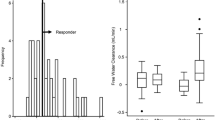
In the TLV group, urine volume per day increased significantly throughout the treatment period. In the FUR group, urine volume increased up to day 2, but did not increase significantly after day 3 (Fig. 1a). The mean change in urine volume over 7 days (or up to the day of early termination) was significantly greater in the TLV group than in the FUR group (p = 0.0029; Fig. 1b). Multiple regression analysis showed that the addition of TLV was a factor predictive of increased urine volume (Table 2). In addition, there was a strong positive correlation between baseline urine osmolality and the change in the urine volume from baseline in the TLV group, but not in the FUR group (Fig. 2a, b). Similarly, a strong positive correlation was also observed between baseline urine specific gravity and the change in the urine volume from baseline in the TLV group, but not in the FUR group (Fig. 2c, d). When subjects were divided into two groups by median baseline urine osmolality (313.5 mOsm/kg), the addition of TLV greatly increased urine volume in the group with high urine osmolality, but there was no difference in the group with low urine osmolality (Fig. 3a, b).
a Correlation between urine osmolality at the start of treatment and the change in urine volume from baseline in the TLV group (n = 14). b Correlation between urine osmolality at the start of treatment and the change in urine volume from baseline in the FUR group (n = 17). c Correlation between urine specific gravity at the start of treatment and the change in urine volume from baseline in the TLV group (n = 15). d Correlation between urine specific gravity at the start of treatment and the change in urine volume from baseline in the FUR group (n = 17)
Secondary end points
Serum creatinine increased from baseline in the FUR group, but not in the TLV group. The incidence of WRF was significantly lower in the TLV group (Fig. 4), with an odds ratio of 0.16 (95% confidence interval 0.03–0.79, p < 0.05). The daily weight change (− 0.58 kg/day vs − 0.39 kg/day; p = 0.057) and the percentage of weight change for 7 days (− 6.37% vs − 4.17%; p = 0.052) showed a trend of decrease in the TLV group compared to the FUR group, but the difference was not significant due to higher water intake in the TLV group (1178 ml/day vs 855 ml/day; p = 0.032). Similarly, leg edema tended to resolve more in the TLV group (80.0% vs 46.7% p = 0.058). TLV significantly decreased BNP (422.8 pg/ml vs 293.1 pg/ml; p = 0.020) and ANP (187.5 pg/ml vs 141.0 pg/ml; p = 0.018). FUR also decreased BNP (520.0 pg/ml vs 409.5 pg/ml; p = 0.025) and ANP (232.8 pg/ml vs 168.2 pg/ml; p = 0.037), with no significant difference between the two groups (BNP: 74.76% vs 79.65%; p = 0.86, ANP: 76.56% vs 76.88%; p = 0.98).
In the TLV group, serum sodium increased on the day after administration, but then showed a downward trend. In contrast, serum sodium in the FUR group decreased consistently (Fig. 5a). In the TLV group, there was a negative correlation between baseline serum sodium and the change in serum sodium during treatment but not in the FUR group (Fig. 5b).
a Alterations in serum Na levels before and after treatment in the TLV and FUR groups. b Correlation between serum sodium levels at the start of treatment and the change in the serum sodium from baseline in the TLV group (n = 16). c Correlation between serum sodium levels at the start of treatment and the change in the serum sodium from baseline in the FUR group (n = 17)
Adverse events
One patient with dry mouth and one with hypernatremia were observed in the TLV group, and one patient with hypotension and two with hyponatremia were observed in the FUR group. No serious adverse events were observed.
Discussion
In patients with advanced CKD who had fluid overload even after treatment with normal doses of FUR, this study showed that the addition of TLV resulted in a greater increase in urine volume and a smaller decrease in renal function than increasing the dose of FUR.
In the conventional comparative studies of TLV and FUR [11, 16, 17], the increase of FUR (often about 20–40 mg/day) in the control group may have been insufficient. Therefore, in this study, the additional dose of FUR in the FUR group was set to 100 mg/day so that the maximum effect of FUR could be expected. Nevertheless, the addition of TLV still achieved a significantly greater increase in urine volume than increasing the dose of FUR.
There was a difference in the baseline dose of FUR between the two groups. However even after adjusting for the baseline dose of FUR in a multivariate analysis, the addition of TLV was an independent factor predictive of increased urine volume. Furthermore, the addition of TLV increased the urine volume irrespective of renal function or responsiveness to FUR.
In our previous retrospective study, we reported that TLV was effective in patients with heart failure who had high urinary osmolality at the start of treatment [12]. Urine osmolality is regulated by ADH. ADH binds to the V2 receptor in the collecting duct of the kidneys and promotes water reabsorption by inducing migration of AQP2 (aquaporin2) to the renal tubule side. ADH is known to be elevated in patients with heart failure, and elevated ADH has been reported to be associated with increased cardiovascular mortality [18]. In patients with heart failure, ADH levels are high despite fluid overload, so it is theoretically possible that TLV, which is a V2 receptor antagonist, would be effective. In particular, patients with high urinary osmolality exhibit relatively insufficient suppression of ADH (excessive ADH), and the effect of TLV is considered to be high in such cases. The present study found that changes in urine volume were strongly correlated with urine osmolality before the start of treatment in the TLV group, but not in the FUR group. Therefore, when we divided patients into two subgroups according to the median urine osmolality, in the group with high urine osmolality, there was a large difference in the change in urine volume between the TLV group and the FUR group (614.1 ml/day, 95% confidence interval 173.4–1054.8), but there was no significant difference in the group with low urine osmolality (285.2 ml/day, 95% confidence interval − 214.5–784.8). From the above, it is considered that TLV addition was more effective than increased FUR dose in increasing the urine volume and expelling excess water from the body, especially in cases in which the urine osmolality at the start of treatment was high.
Measurement of urine osmolality was shown to be useful in the selection of diuretics. However, measuring urine osmolality is often time consuming and may not be measured in the clinic. On the other hand, the urine specific gravity can be measured immediately and easily in the clinic using only a test paper, without the need for special equipment. In this study, we found that the higher the urine specific gravity, the higher the effect of TLV, as in the case of urine osmolality. This would constitute an additional advantage of TLV treatment—namely, its therapeutic effect can be predicted simply by a urine specific gravity test.
It has been reported that renal dysfunction is often associated with the treatment process of fluid excess [19]. WRF may be diagnosed when treatment increases serum creatine levels by 0.3 mg/dl or more, and WRF is known to be a poor prognostic factor [20]. In the treatment of heart failure and renal failure, it is important to stabilize the condition without causing WRF. WRF was thought to be caused by hypoperfusion of the kidneys due to decreased cardiac output and decreased intravascular volume due to diuretic use [21]. However, venous stasis was recently reported to be the factor most associated with WRF [22]. Multiple mechanisms are now thought to contribute to the development of WRF, including initiation of angiotensin II receptor blocker (ARB) / angiotensin-converting enzyme inhibitor (ACEI) [23] and hypotension [24]. Kin et al. reported that in heart failure patients who did not have impaired renal function, treatment with TLV reduced the risk of developing WRF compared with treatment with FUR [25]. Our present study, which included patients with advanced CKD, also found that TLV was less likely to cause WRF than FUR. Whereas FUR mainly reduces extracellular fluid, the combination therapy of TLV and FUR has been reported to reduce intracellular fluid and extracellular fluid as much, resulting in relatively preserved renal blood flow [17]. In addition, several studies [11, 26] have reported that combination therapy improved congestion more, and these may be the factors that prevented the development of WRF. Therefore, TLV was safer, and appeared to improve the prognosis of kidney failure in addition to that of heart failure.
It has been pointed out that patients with heart failure are more likely to develop hyponatremia because they are often treated with diuretics and a low-salt diet [27]. Hyponatremia is considered to be one of the important causes of poor prognosis in patients with heart failure [28], and thus prevention of its onset is important. FUR has been reported to cause hyponatremia in a dose-dependent manner [29], and unfortunately, the serum sodium concentration was significantly decreased by increasing the dose of FUR in this study as well. On the other hand, with the addition of TLV, which is a water diuretic, mild elevation of serum sodium was observed in patients with a tendency toward hyponatremia. Although sufficient caution is required for the development of hypernatremia, no cases with severe hypernatremia were found in this study. Combination treatment with a loop diuretic and TLV can be expected to avoid the hyponatremia observed by treatment with loop diuretics.
Limitations
This study has several limitations. First, although the study period was extended, the target number of cases was still not reached. There was a tendency for the TLV group to lose more weight and improve leg edema more than the FUR group, but the difference was not significant. Increasing the number of cases may have made a difference. Second, the alterations in BNP and ANP were not significantly different between the two groups. Further studies are needed to determine whether TLV improves heart failure better than FUR. Third, most of the patients were HFpEF patients (88% in the TLV group and 76% in the FUR group). Therefore, we could not determine whether similar results would be obtained in HFrEF patients with advanced CKD. Fourth, since it is also interested in the long-term cardioprotective effect of TLV, but we cannot find the answer because this study examines the short-term effect of TLV on advanced CKD. Long-term studies are required in the future.
Conclusion
In patients with advanced CKD with fluid overload, addition of TLV achieved a significantly greater increase in urine volume compared to increasing the dose of FUR, and TLV addition did not increase the adverse effects on renal function.
The efficacy and safety of TLV were higher in our patients with higher urine osmolality, and were also higher in our patients with lower serum sodium before the start of treatment.
Patients with advanced CKD usually experience electrolyte imbalance, and their kidneys cannot easily compensate for sudden changes in circulating blood volume. Careful introduction of TLV in patients with CKD with heart failure may improve cardiorenal associations and patient prognosis and quality of life, and thus is an important treatment option.
References
De Bruyne LK. Mechanisms and management of diuretic resistance in congestive heart failure. Postgrad Med J. 2003;79(931):268–71.
Andreucci M, Russo D, Fuiano G, Minutolo R, Andreucci VE. Diuretics in renal failure. Miner Electrolyte Metab. 1999;25(1–2):32–8.
Felker GM, O’Connor CM, Braunwald E. Heart Failure Clinical Research Network Investigators. Loop diuretics in acute decompensated heart failure: Necessary? Evil? A necessary evil? Circ Heart Fail. 2009;2(1):56–62.
Miyazaki T, Fujiki H, Yamamura Y, Nakamura S, Mori T. Tolvaptan, an orally active vasopressin V(2)-receptor antagonist—pharmacology and clinical trials. Cardiovasc Drug Rev. 2007;25(1):1–13.
Gheorghiade M, Konstam MA, Burnett JC Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297(12):1332–43.
Matsuzaki M, Hori M, Izumi T, Fukunami M, Tolvaptan Investigators. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study). Cardiovasc Drugs Ther. 2011;25(Suppl 1):S33–45.
Kinugawa K, Sato N, Inomata T, Shimakawa T, Iwatake N, Mizuguchi K. Efficacy and safety of tolvaptan in heart failure patients with volume overload [published correction appears in Circ J. 2014;78(7):1773]. Circ J. 2014;78(4):844–52.
Sen J, Chung E, McGill D. Tolvaptan for heart failure in chronic kidney disease patients: a systematic review and meta-analysis. Heart Lung Circ. 2018;27(8):928–39.
Ikeda S, Ohshima K, Miyazaki S, et al. Impact of chronic kidney disease on the diuretic response of tolvaptan in acute decompensated heart failure. ESC Heart Fail. 2017;4(4):614–22.
Matsue Y, Ter Maaten JM, Suzuki M, et al. Early treatment with tolvaptan improves diuretic response in acute heart failure with renal dysfunction. Clin Res Cardiol. 2017;106(10):802–12.
Tanaka T, Minatoguchi S, Yamada Y, et al. Addition of tolvaptan compared with increased dose of furosemide in heart failure patients With chronic kidney disease under furosemide treatment. Circ Rep. 2018;1(1):35–41 (Published 2018 Dec 12).
Katsumata M, Hirawa N, Sumida K, et al. Effects of tolvaptan in patients with chronic kidney disease and chronic heart failure. Clin Exp Nephrol. 2017;21(5):858–65.
Tominaga N, Kida K, Matsumoto N, et al. Safety of add-on tolvaptan in patients with furosemide-resistant congestive heart failure complicated by advanced chronic kidney disease: a sub-analysis of a pharmacokinetics/ pharmacodynamics study. Clin Nephrol. 2015;84(1):29–38.
Japan Nephrology Society Special issue. Clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012. Nihon Jinzo Gakkai Shi. 2012;54:1034–191.
Group Leader Matsuzaki M. Guidelines for the Diagnosis and Treatment of Cardiovascular Disease, Guidelines for Treatment of Chronic Heart Failure (JCS 2010). http://www.shiga-med.ac.jp/~hqeiyo/CHF2010.pdf. Accessed 15 Apr 2014.
Inomata T, Ikeda Y, Kida K, et al. Effects of additive tolvaptan vs. increased furosemide on heart failure with diuretic resistance and renal impairment—results from the K-STAR study. Circ J. 2017;82(1):159–67.
Takagi K, Sato N, Ishihara S, et al. Differences in pharmacological property between combined therapy of the vasopressin V2-receptor antagonist tolvaptan plus furosemide and monotherapy of furosemide in patients with hospitalized heart failure. J Cardiol. 2020;76(5):499–505.
Ambrosy A, Goldsmith SR, Gheorghiade M. Tolvaptan for the treatment of heart failure: a review of the literature. Expert Opin Pharmacother. 2011;12(6):961–76.
Brandimarte F, Vaduganathan M, Mureddu GF, et al. Prognostic implications of renal dysfunction in patients hospitalized with heart failure: data from the last decade of clinical investigations. Heart Fail Rev. 2013;18(2):167–76.
Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35(7):455–69.
Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs. 1990;39(Suppl 4):10–24.
Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–96.
Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail. 2011;4(6):685–91.
Testani JM, Coca SG, McCauley BD, Shannon RP, Kimmel SE. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail. 2011;13(8):877–84.
Kin H, Matsumura K, Yamamoto Y, et al. Renoprotective effect of tolvaptan in patients with new-onset acute heart failure. ESC Heart Fail. 2020;7(4):1764–70.
Matsue Y, Suzuki M, Torii S, et al. Clinical Effectiveness of Tolvaptan in Patients With Acute Heart Failure and Renal Dysfunction [published correction appears in J Card Fail. 2016 Nov;22(11):941]. J Card Fail. 2016;22(6):423–32.
Gheorghiade M, Abraham WT, Albert NM, et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28(8):980–8.
Oren RM. Hyponatremia in congestive heart failure. Am J Cardiol. 2005;95(9A):2B-7B.
Velat I, Bušić Ž, Jurić Paić M, Čulić V. Furosemide and spironolactone doses and hyponatremia in patients with heart failure. BMC Pharmacol Toxicol. 2020;21(1):57.
Funding
This work was supported by the Grants for Research from Yokohama City University, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Honoraria: Koichi Tamura (Takeda, AstraZeneca, Daiichi-Sankyo, Novartis), Nobuhito Hirawa (Takeda), Grants received: Koichi Tamura (Bayer, Takeda, Daiichi-Sankyo, AstraZeneca, Ono Pharmaceutical, Kyowa Kirin, Novartis).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB approval number D1405025) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Komiya, S., Katsumata, M., Ozawa, M. et al. Efficacy of tolvaptan on advanced chronic kidney disease with heart failure: a randomized controlled trial. Clin Exp Nephrol 26, 851–858 (2022). https://doi.org/10.1007/s10157-022-02224-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-022-02224-x