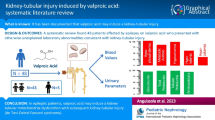
Abstract
Objective
To explore the risk factors for the development of sodium valproate (VPA)-induced renal tubular dysfunction for early diagnosis and treatment.
Study design
The subjects were selected from patients who were diagnosed with epilepsy and administered VPA. Blood and spot urine samples were collected and measured the concentration of VPA, the level of serum phosphorus, serum uric acid, serum free carnitine, serum cystatin-c, and urine β2-microglobulin (BMG). Patients with urine BMG/creatinine levels above 219.2 were treated as renal proximal tubular dysfunction (RTD), with all others treated as non-RTD.
Results
Eighty-seven patients, 4–48 years, 53 men and 34 women, were studied. RTD group is 17 patients and non-RTD group is 70 patients. Univariate analyses revealed that the RTD patients were more likely to be bedridden, receiving enteral tube feeding, taking more anticonvulsants, and demonstrating significantly lower serum levels of free carnitine, uric acid, and phosphorus. Among them, bedridden, free serum carnitine, and phosphorus levels were associated with the development of RTD by multivariate analysis.
Conclusions
Bedridden patients receiving VPA are susceptible to hypocarnitinemia, which can cause RTD and may lead to FS. Therefore, urinary BMG should be measured regularly in all patients receiving VPA to assess renal tubular function. An additional measurement of serum free carnitine level should be considered in patients who developed RTD. Supplementation of carnitine for those patients to prevent such complication deserves for further study.

Similar content being viewed by others
Abbreviations
- FS:
-
Fanconi syndrome
- VPA:
-
Sodium valproate
- BMG:
-
β2-microglobulin
- Cr:
-
Creatinine
- PTCs:
-
Proximal tubular cells
- RTD:
-
Renal proximal tubular dysfunction
References
Hall AM, Bass P, Unwin RJ. Drug-induced renal Fanconi syndrome. Q J Med. 2014;107:261–9.
Izzedine H, Launay-Vacher V, Isnard-Bagnis C, Deray G. Drug-induced Fanconi’s syndrome. Am J Kidney Dis. 2003;41:292–309.
Dhillon N, Högler W. Fractures and Fanconi syndrome due to prolonged sodium valproate use. Neuropediatrics. 2011;42:119–21.
Patel SM, Graff-Radford J, Wieland ML. Valproate-induced Fanconi syndrome in a 27-year-old woman. J Gen Intern Med. 2011;26:1072–4.
Inoue T, Tanaka Y, Otani R, Itabashi H, Murakami N, Nagai T, et al. Three cases of Fanconi syndrome associated with valproate sodium treatment. No To Hattatsu. 2011;43:233–7.
Yoshikawa H, Yamazaki S, Abe T. Hypouricemia in severely disabled children: influence of elemental enteral nutrition on the serum uric acid levels. Brain Dev. 2004;26:43–6.
Takahashi M, Ueda S, Misaki H, Sugiyama N, Matsumoto K, Matsuo N, et al. Carnitine determination by an enzymatic cycling method with carnitine dehydrogenase. Clin Chem. 1994;40:817–21.
Madsen MG, Nørregaard R, Palmfeldt J, Olsen LH, Frøkiær J, Jørgensen TM. Urinary NGAL, cystatin C, β2-microglobulin, and osteopontin significance in hydronephrotic children. Pediatr Nephrol. 2012;27:2099–106.
Takayanagi M. Abnormalities of carnitine metabolism in human diseases. J Anal Bio-Sci. 2012;35:281–92.
Lenoir GR, Perignon JL, Gubler MC, Broyer M. Valproic acid: a possible cause of proximal tubular renal syndrome. J Pediatr. 1981;98:503–4.
Yoshikawa H, Watanabe T, Abe T. Tubulo-interstitial nephritis caused by sodium valproate. Brain Dev. 2002;24:102–5.
Yoshikawa H, Watanabe T, Abe T. Fanconi syndrome caused by sodium valproate: report of three severely disabled children. Eur J Paediatr Neurol. 2002;6:165–7.
Zaki EL, Springate JE. Renal injury from valproic acid: case report and literature review. Pediatr Neurol. 2002;27:318–9.
Fukuda Y, Watanabe H, Ohtomo Y, Yabuta K. Immunologically mediated chronic tubulo-interstitial nephritis caused by valproate therapy. Nephron. 1996;72:328–9.
Keith KL, Koyelle P. Anticonvulsant-induced rickets and nephrocalcinosis. BMJ Case Rep. 2012;. doi:10.1136/bcr.12.2011.5359.
Shiari R, Bagherzade L, Alaei MR. Fanconi syndrome associated with valporic Acid: a case report. Iran Red Crescent Med J. 2011;13:844–5.
Tanaka S, Suzukawa J, Araki A, Imai Y, Takaya J, Taniuchi S, et al. Hypouricemic acute renal failure in a patient with valproate-induced Fanconi syndrome. Jpn J Pediatr Nephrol. 2008;21:182–7.
Knights MJ, Finlay E. The effects of sodium valproate on the renal function of children with epilepsy. Pediatr Nephrol. 2010;29:1131–8.
Endo A, Fujita Y, Fuchigami T, Takahashi S, Mugishima H. Fanconi syndrome caused by valproic acid. Pediatr Neurol. 2010;42:287–90.
Yamazaki S, Watanabe T, Sato S, Yoshikawa H. Outcomes of renal proximal tubular dysfunctions with Fanconi syndrome caused by sodium valproate. Pediatr Int. 2016;58:1023–6.
Watanabe T, Yoshikawa H, Yamazaki S, Abe Y, Abe T. Secondary renal Fanconi syndrome caused by valproate therapy. Pediatr Nephrol. 2005;20:814–7.
Hawkins E, Brewer E. Renal toxicity induced by valproic acid (Depakene). Pediatr Pathol. 1993;13:863–8.
Knorr M, Schaper J, Harjes M, Mayatepek E, Rosenbaum T. Fanconi syndrome caused by antiepileptic therapy with valproic Acid. Epilepsia. 2004;45:868–71.
Igarashi N, Sato T, Kyouya S. Secondary carnitine deficiency in handicapped patients receiving valproic acid and/or elemental diet. Acta Paediatr Jpn. 1990;32:139–45.
Bremer J. Carnitine-metabolism and functions. Phys Rev. 1983;63:1420–79.
Rebouche CJ. Carnitine function and requirements during the life cycle. FASEB J. 1992;6:3379–86.
Kato A. Carnitine deficiency with Valproate sodium therapy—the difference by normal diet and enteral nutrition. No To Hattatsu. 2013;45:17–20.
Silva MF, Aires CC, Luis PB, Ruiter JP, IJlst L, et al. Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: a review. J Inherit Metab Dis. 2008;31:205–16.
Philippe ERL, Andrea P, Soheil Z, Mireille G. Science review: carnitine in the treatment of valproic acid-induced toxicity—what is the evidence? Crit Care. 2005;9:431–40.
Nagata J. Basics of carnitine in health foods: analysis, biochemistry and physiological functions. Int J Anal Bio-Sci. 2012;35:275–80.
Lin CY, Chiang H. Sodium-valproate-induced interstitial nephritis. Nephron. 1988;48:43–6.
Raza M, Al-Bekairi AM, Ageel AM, Qureshi S. Biochemical basis of sodium valproate hepatotoxicity and renal tubular disorder: time dependence of peroxidative injury. Pharmacol Res. 1997;35:153–7.
Acknowledgements
We are grateful to the members of the Department of Pediatrics at Kansai Medical University for their important contributions to the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB approval number 26–30, H141172) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
About this article
Cite this article
Koga, S., Kimata, T., Yamanouchi, S. et al. Risk factors for sodium valproate-induced renal tubular dysfunction. Clin Exp Nephrol 22, 420–425 (2018). https://doi.org/10.1007/s10157-017-1472-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1472-z