Abstract
The mortality of acute kidney injury (AKI) remains unacceptably high, especially associated with acute respiratory failure. Lung injury complicated with AKI was previously considered as “uremic lung”, which is characterized by volume overload and increased vascular permeability. New experimental data using rodent models of renal ischemia–reperfusion and bilateral nephrectomy have emerged recently focusing on kidney–lung crosstalk in AKI, and have highlighted the pathophysiological significance of increased cytokine concentration, enhanced inflammatory responses, and neutrophil activation. In this review, we outline the history of uremic lung and acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), the epidemiological data on the synergistic effect of AKI and lung injury on mortality, and recent basic research which has identified possible pathways in AKI-induced lung injury. These findings will enable us to develop new therapeutic strategies against lung injury associated with AKI and improve the outcomes of critically ill patients in intensive care units.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is a severe complication for critically ill patients in intensive care units (ICUs). It has been reported that AKI markedly increases their mortality [1–4], although renal dysfunction in AKI can be adequately treated by renal replacement therapy (RRT). This indicates the necessity of new AKI therapeutic strategies to improve the outcomes of AKI patients, targeting not only renal dysfunction alone but also multiple organ failure. Remote organ injury induced by AKI has recently been suggested [5]; for instance, AKI induces cardiac cell apoptosis and interleukin (IL)-1 and tumor necrosis factor (TNF)-α production in a rat renal ischemia–reperfusion model [6]. AKI increases proinflammatory cytokines in the cerebral cortex and hippocampus, activates microglia, and influences locomotor activity in mice [7].
Acute respiratory failure is another life-threatening condition in ICU patients. The mortality of acute respiratory failure is higher than 50% and increases with the development of other organ failure including AKI [8]. One report described a mortality rate above 80% for patients with AKI complicated with acute respiratory failure [1]. Therefore, understanding the pathophysiology of lung injury associated with AKI (kidney–lung crosstalk) and development of a new treatment for AKI complicated with acute lung injury will be crucial to improving the outcomes of AKI. In this review, we outline pulmonary complications in AKI, clinical epidemiological data on acute respiratory failure complicated with AKI, and recent basic research which has identified possible pathways in AKI-induced lung injury.
Uremic lung in AKI
“Uremic lung” is a term that describes pulmonary complication observed in uremic patients. Lange described this condition in the literature for the first time and the chest X-ray findings that are characterized by hilar opacities with a clear periphery were reported subsequently in the 1930s (Table 1) [9, 10]. The pathophysiology of uremic lung was assumed to be due to volume overload and increased capillary leakage, possibly caused by uremic substances. However, some groups observed inflammatory changes in the pulmonary lesions in uremic patients. Hopps and Wissler [11] examined lung pathology in autopsy samples from 107 uremic patients and 429 non-uremic patients. They found hyaline membrane formation and inflammatory cell exudates in the alveoli and described these pathological changes as uremic pneumonitis. Rackow and Fein [12] examined pulmonary capillary wedge pressure and bronchoalveolar lavage (BAL) fluid in two AKI patients. They found no volume overload and increased BAL fluid protein concentration, indicating that fluid overload is not necessary to cause pulmonary lesions in uremic patients. Bleyl et al. [13] examined 66 autopsy cases and reported that hyaline membrane formation was found not just in chronic renal failure but in all AKI patients. Physicians now recognize that the pulmonary edema found in end-stage renal disease (ESRD) patients can be rapidly resolved by dialysis induction, while patients with acute respiratory failure complicated with AKI show markedly higher mortality even if treated by dialysis [14, 15]. Taken together, not only fluid overload and increased vascular permeability but also inflammatory changes contribute to the pathogenesis of uremic lung, especially in AKI.
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) was first described in 1967 by Ashbaugh et al. [16]. Twelve patients showed acute respiratory distress, cyanosis refractory to oxygen therapy, decreased lung compliance, and diffuse pulmonary infiltrates on chest X-ray. Historically, this disorder was frequently observed in the victims of war and recognized as post-traumatic lung (DaNang Lung) [17]. In 1994 a new definition was recommended by the American–European Consensus Conference Committee [18]. ALI/ARDS is defined by the four criteria listed in Table 2. Although formally considered different from ARDS, ALI is now recognized as the less severe form, which is defined by the same parameters except for a PaO2/FiO2 ratio between 201 and 300. There are two stages in ALI/ARDS: the acute phase is characterized by disruption of the alveolar capillary and epithelial barrier, leakage of protein-rich fluid into the interstitium and alveolar space (hyaline membrane formation), and extensive release of cytokines and neutrophil infiltration. The later phase is characterized by fibrosis formation and permanent lung scarring [19, 20].
A number of conditions can cause ALI/ARDS directly (pulmonary injury) and indirectly (systemic injury) (Table 3). The most frequent direct causes are pneumonia and aspiration of gastric contents, whereas the most common causes of indirect injury are sepsis and trauma. Although AKI is not widely considered as a cause of ALI/ARDS, AKI shares common indirect factors such as sepsis, trauma, hemorrhage, and acute pancreatitis with ALI/ARDS. Recent basic researches using the rodent AKI models described below suggest that AKI could be another indirect cause of ALI/ARDS. Further investigation is necessary to demonstrate the kidney–lung crosstalk in the clinical setting of ALI complicated with AKI.
Mortality of ALI/ARDS and AKI
ALI/ARDS and AKI will synergistically worsen the outcome of ICU patients. Russell et al. [21] reported that pulmonary dysfunction is common in sepsis syndrome, but not associated with an increased mortality rate. However, they found an increase in mortality with a pattern characterized by worsening renal dysfunction over the first 3 days; development of AKI in respiratory failure patients indicated poor outcomes. Chertow et al. [1] analyzed a clinical cohort of severe AKI patients who required dialysis and observed a markedly higher mortality rate in mechanically ventilated patients (81%) compared with the rest (29%). In an observational cohort study of AKI patients in ICUs at five academic medical centers (the Program to Improve Care in Acute Renal Disease; PICARD study), complications of respiratory failure significantly increased the mortality of AKI patients with an odds ratio of 2.62 (95% confidence interval [CI] 1.70–4.04) [22]. Liu et al. [23] analyzed 876 ARDS patients enrolled in the National Heart, Lung, and Blood Institute ARDS Clinical Network trial. Thirty-five percent of ARDS patients developed AKI and the mortality rate significantly increased with AKI complications (odds ratio 3.36, 95%CI 2.35–4.81). Vieira et al. examined 140 cancer patients who needed mechanical ventilation for acute respiratory failure. They found that AKI had a significant impact on not only the mortality but also the duration of mechanical ventilation and weaning from mechanical ventilation [24]. These clinical data strongly indicate that mortality will be unacceptably high when ICU patients have complications of AKI and ALI/ARDS.
Basic research on AKI-induced lung injury
Recently, it has been suggested that AKI influences other remote organs including the lungs [5, 15, 25]. Experimental evidence indicates that AKI-induced lung injury occurs not only by volume overload but also through deleterious kidney–lung interactions. It is of note that the alveolar epithelium has common features with the renal tubular epithelium in terms of polarization (apical and basolateral sites), localization of channels and transporters, and existence of tight junctional complexes (Fig. 1). Mechanisms of AKI-induced lung injury are assumed to include dysregulation of water clearance, inflammatory reaction, innate immune response, oxidative stress, apoptosis, and soluble mediator metabolism [26].
Rabb and colleagues [27, 28] reported that ischemic AKI engenders increased pulmonary vascular permeability accompanied by down-regulation of the pulmonary epithelial sodium channel (ENaC), Na,K-ATPase, and aquaporin-5. Interestingly, these changes were found, not in unilateral ischemia with an intact contralateral kidney, but in bilateral nephrectomy (BNx). They concluded that down-regulation of water clearance molecules in the lung might be mediated by unknown uremic toxins rather than reperfusion products. Ischemic AKI induces inflammatory reactions in the lung. Star and colleagues demonstrated activation of inflammatory transcription factors such as nuclear factor-κB and p38 mitogen-activated protein kinase in the lung. They also showed the protective effect of α-melanocyte stimulating hormone (α-MSH) on AKI-induced lung injury [29]. α-MSH is a potent anti-inflammatory cytokine that decreases inflammatory cytokines, nitric oxide production, and expression of a neutrophil adhesion molecule [30, 31]. α-MSH inhibits multiple forms of inflammation and shows protective effects on AKI induced by ischemia–reperfusion and cisplatin injection [32–35].
Faubel and colleagues reported that AKI after ischemia and BNx induced increases in multiple serum cytokines, including IL-6 and IL-1β, and lung injury characterized by neutrophil infiltration. The anti-inflammatory cytokine IL-10 attenuated pulmonary neutrophil infiltration and BAL fluid protein levels [36]. They further demonstrated the role of IL-6 in lung injury associated with AKI using IL-6-deficient mice and neutralizing antibody against IL-6 [37]. Rabb and colleagues [38, 39] recently performed a comprehensive gene expression analysis to clarify the response of the lung to AKI. Gene chip analysis identified 266 lung genes with increased expression and 615 with decreased expression at 6 h after surgery of renal ischemia reperfusion. Interestingly, the influence of BNx on pulmonary gene transcription was undetectable at 6 h. Gene ontology analysis demonstrated activation of several proinflammatory and proapoptotic biological processes. They also found that inflammatory transcriptome changes in the kidney and the lung were similar and included the innate immunity genes such as Cd14, Socs3, Saa3, and Lcn2 (lipocalin 2).
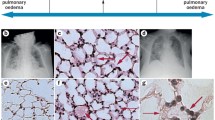
Neutrophil infiltration into the lung is a key event, especially in the early phase of lung injury associated with AKI. Okusa and colleagues reported an increase in lung neutrophil content following ischemic AKI in mouse [40]. They showed a significant increase in marginal neutrophils after ischemic AKI but no significant change in the lung interstitial neutrophil content. These data suggest that transmigration of neutrophils to the alveolar space is not necessary for inducing lung injury in AKI. Activated neutrophils contribute to the pathogenesis of lung injury by releasing several proteases and reactive oxygen species [41]. Neutrophil elastase (NE) is a serine protease secreted by neutrophils during inflammation and destroys not only bacteria but also host tissue [42, 43]. Recently, we evaluated the possible therapeutic effects of a specific NE inhibitor, sivelestat sodium hydrate (ONO-5046, Elaspol™), on lung injury associated with AKI using a mouse BNx model [44]. BNx showed neutrophil infiltration into the lung (Fig. 2), increased pulmonary inflammatory cytokine expression (IL-6, neutrophil chemokine keratinocyte-derived chemokine [KC] and TNF-α), and protein leakage accompanied by early increase of systemic and pulmonary NE activity. The sivelestat sodium hydrate treatment reduced systemic NE activity and improved these pulmonary inflammatory responses. Although one randomized controlled trial including 492 patients demonstrated no improvement in ALI/ARDS by sivelestat sodium hydrate [45], several small trials showed beneficial effects of sivelestat sodium hydrate on ALI/ARDS in critically ill patients [46, 47]. No clinical trial evaluating sivelestat sodium hydrate on ALI/ARDS complicated with AKI has been reported in the relevant literature. This study suggests that blockade of NE activity may be a useful therapeutic strategy for lung injury complication in AKI patients. On the contrary, one study employing a murine two-hit model consisted of AKI (ischemia–reperfusion or BNx) and subsequent ALI (intratracheal HCl instillation), demonstrated that acute renal dysfunction was protective for subsequent lung injury [48]. Reconstitution experiments, in which uremic neutrophils were injected into non-uremic mice and vice versa, identified uremic neutrophils as the primary mediators. Further investigations are necessary to clarify the role of neutrophils in the pathogenesis of lung injury associated with AKI.
Although investigations of AKI-induced lung injury have been conducted by several different groups as described above, there are few animal studies that have examined ALI-induced AKI. Considering the high frequency of AKI complications in ARDS patients [23], there is assumed to be AKI induction by ALI. Slutsky and colleagues [49] reported a rabbit model of acid-aspiration lung injury followed by mechanical ventilation with injurious ventilatory strategies (high tidal volume + high PEEP) which showed increased rates of epithelial cell apoptosis in the kidney. On the other hand, Simon and colleagues found no renal structural and functional alterations in a canine acid-aspiration model of ALI when hemodynamics and arterial blood gas tensions are carefully controlled [50]. It is of note that only aseptic ALI models were used for these studies.
Limitation of animal models
Because failure to translate benefits observed in animal models to the clinical setting is a huge problem, development of clinically relevant animal models is essential [51]. As described above, rodent models of BNx and renal ischemia–reperfusion have been employed to investigate the role of AKI in the pathogenesis of lung injury [27, 29, 36, 37]. Both animal models have some disadvantages. As with renal ischemia, ischemia–reperfusion models of other organs such as the liver, gut, and hind limb also cause lung injury [52–54]. Intestinal ischemia–reperfusion injury induced marked lung injury, as characterized by lung edema, hemorrhage, and infiltration of inflammatory cells, and proinflammatory cytokine (IL-6 and TNF-α) levels in the lung [55]. Similarly, pulmonary edema has been observed within hours of liver transplantation [56, 57], which has been attributed to hepatic ischemia–reperfusion injury via the activation of the hepatic Kupffer cells resulting in the production of oxygen radicals and the modulation of proinflammatory and anti-inflammatory cytokines [58–60]. The secondary organ damage after ischemia–reperfusion in different organ is caused by systemic induction of oxygen radicals and proinflammatory cytokines rather than the loss of organ function. Therefore, it would be difficult to distinguish the contribution of renal dysfunction itself in lung injury from the effects derived from increased systemic oxidative stress. Because the BNx model induces abrupt and complete decline of renal function, it ideally enables us to investigate the direct relation of renal dysfunction with lung injury without considering the additional issues described above. However, the BNx model is not perfectly relevant to clinical settings because sudden and complete loss of renal function will not be seen so frequently. Kidney cancer patients who have already lost one kidney for some reason and have total nephrectomy, or victims of traumatic accidents, may suffer from this condition.
Reportedly, a mouse model of AKI-induced lung injury using BNx showed no volume overload or pulmonary edema [36, 44]. No significant difference was found between bilaterally nephrectomized and sham-operated animals in terms of total body weight change or the lung wet/dry weight ratio. Severely ill patients with AKI-induced lung injury frequently show lung edema, possibly because they need fluid resuscitation to maintain their hemodynamics. However, these studies administered no fluid to the animals after the surgery. Although marked inflammatory responses including neutrophil infiltration, increased vascular permeability, and inflammatory cytokine expression were demonstrated in the BNx model, adding fluid as resuscitation will produce an AKI-induced lung injury model closer to the clinical settings.
Perspective
ALI/ARDS and AKI synergistically worsen the outcome of critically ill patients, and development of novel therapeutic strategies against these severe conditions will certainly make marked progress in the field of critical care. Basic research has recently demonstrated that lung injury can be induced by AKI and has indicated the possible existence of kidney–lung crosstalk. Several different pathways have been identified that contribute to the lung injury following AKI: dysfunction in water clearance, neutrophil activation, increase in cytokine concentration, activation of inflammatory transcription factors, and induction of inflammatory gene expressions. Although we are accustomed to having a single fixed therapeutic target (e.g., HMG-CoA reductase for hypercholesterolemia), the therapeutic targets in lung injury following AKI will vary and may need new biomarkers to identify which biological targets are active at any given time in the patients.
Lung injury is induced not only by renal ischemia–reperfusion but by BNx in rodent models, indicating that an unknown “uremic toxin” which is eliminated by the kidney may cause lung injury. This raises questions whether chronic kidney disease and ESRD are the risks for acute lung injury, and whether RRT can ameliorate AKI-induced lung injury by removing unknown substances. Can AKI or renal dysfunction itself be recognized as a cause of ALI/ARDS (Table 3)? Can we improve the ventilator-free days by starting RRT earlier or conducting high-flow, high-volume continuous RRT? Clinical research focused on these issues will further our understanding of kidney–lung crosstalk.
References
Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155:1505–11.
Chertow GM, Lazarus JM, Paganini EP, Allgren RL, Lafayette RA, Sayegh MH. Predictors of mortality and the provision of dialysis in patients with acute tubular necrosis. The Auriculin Anaritide Acute Renal Failure Study Group. J Am Soc Nephrol. 1998;9:692–8.
Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051–8.
Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol. 2002;13:1350–7.
Scheel PJ, Liu M, Rabb H. Uremic lung: new insights into a forgotten condition. Kidney Int. 2008;74:849–51.
Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:1549–58.
Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–70.
Liu KD, Matthay MA. Advances in critical care for the nephrologist: acute lung injury/ARDS. Clin J Am Soc Nephrol. 2008;3:578–86.
Lange W. Uber eine eigentumliche Erkrankung der kleinen Bronchien obliterans. Dtsch Arch Klin Med. 1901;70:342–64.
Roubier CH, Plauchu M. Sur certain aspects radiographiques de l’oedeme pulmonaire chez les cardio-renaux. Lyon méd. 1933;152:137.
Hopps HC, Wissler RW. Uremic pneumonitis. Am J Pathol. 1955;31:261–73.
Rackow EC, Fein IA. Fulminant noncardiogenic pulmonary edema in the critically ill. Crit Care Med. 1978;6:360–3.
Bleyl U, Sander E, Schindler T. The pathology and biology of uremic pneumonitis. Intensive Care Med. 1981;7:193–202.
Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–69.
Faubel S. Pulmonary complications after acute kidney injury. Adv Chronic Kidney Dis. 2008;15:284–96.
Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–23.
Oud L. From DaNang lung to combat trauma-associated acute lung injury-closing the loop. South Med J. 2009;102:567–8.
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24.
Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49.
Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–49.
Russell JA, Singer J, Bernard GR, Wheeler A, Fulkerson W, Hudson L, et al. Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit Care Med. 2000;28:3405–11.
Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–21.
Liu KD, Glidden DV, Eisner MD, Parsons PE, Ware LB, Wheeler A, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007;35:2755–61.
Vieira JM Jr, Castro I, Curvello-Neto A, Demarzo S, Caruso P, Pastore L Jr, et al. Effect of acute kidney injury on weaning from mechanical ventilation in critically ill patients. Crit Care Med. 2007;35:184–91.
Feltes CM, Van Eyk J, Rabb H. Distant-organ changes after acute kidney injury. Nephron Physiol. 2008;109:p80–4.
Ko GJ, Rabb H, Hassoun HT. Kidney–lung crosstalk in the critically ill patient. Blood Purif. 2009;28:75–83.
Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int. 2003;63:600–6.
Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55:2362–7.
Deng J, Hu X, Yuen PS, Star RA. Alpha-melanocyte-stimulating hormone inhibits lung injury after renal ischemia/reperfusion. Am J Respir Crit Care Med. 2004;169:749–56.
Star RA, Rajora N, Huang J, Stock RC, Catania A, Lipton JM. Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci USA. 1995;92:8016–20.
Kohda Y, Chiao H, Star RA. Alpha-melanocyte-stimulating hormone and acute renal failure. Curr Opin Nephrol Hypertens. 1998;7:413–7.
Chiao H, Kohda Y, McLeroy P, Craig L, Housini I, Star RA. Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J Clin Invest. 1997;99:1165–72.
Kwon TH, Frokiaer J, Fernandez-Llama P, Knepper MA, Nielsen S. Reduced abundance of aquaporins in rats with bilateral ischemia-induced acute renal failure: prevention by alpha-MSH. Am J Physiol. 1999;277:F413–27.
Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, et al. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–28.
Gong H, Wang W, Kwon TH, Jonassen T, Li C, Ring T, et al. EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney. Kidney Int. 2004;66:683–95.
Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18:155–64.
Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008;74:901–9.
Hassoun HT, Grigoryev DN, Lie ML, Liu M, Cheadle C, Tuder RM, et al. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol Renal Physiol. 2007;293:F30–40.
Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008;19:547–58.
Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, et al. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 2009;75:689–98.
Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–76.
Belaaouaj A, Kim KS, Shapiro SD. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science. 2000;289:1185–8.
Chua F, Laurent GJ. Neutrophil elastase: mediator of extracellular matrix destruction and accumulation. Proc Am Thorac Soc. 2006;3:424–7.
Ishii T, Doi K, Okamoto K, Imamura M, Dohi M, Yamamoto K, et al. Neutrophil elastase contributes to acute lung injury induced by bilateral nephrectomy. Am J Pathol. 2010;177:1665–73.
Zeiher BG, Artigas A, Vincent JL, Dmitrienko A, Jackson K, Thompson BT, et al. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med. 2004;32:1695–702.
Akamoto S, Okano K, Sano T, Yachida S, Izuishi K, Usuki H, et al. Neutrophil elastase inhibitor (sivelestat) preserves antitumor immunity and reduces the inflammatory mediators associated with major surgery. Surg Today. 2007;37:359–65.
Inoue Y, Tanaka H, Ogura H, Ukai I, Fujita K, Hosotsubo H, et al. A neutrophil elastase inhibitor, sivelestat, improves leukocyte deformability in patients with acute lung injury. J Trauma. 2006;60:936–43. discussion 943.
Zarbock A, Schmolke M, Spieker T, Jurk K, Van Aken H, Singbartl K. Acute uremia but not renal inflammation attenuates aseptic acute lung injury: a critical role for uremic neutrophils. J Am Soc Nephrol. 2006;17:3124–31.
Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–12.
Hoag JB, Liu M, Easley RB, Britos-Bray MF, Kesari P, Hassoun H, et al. Effects of acid aspiration-induced acute lung injury on kidney function. Am J Physiol Renal Physiol. 2008;294:F900–8.
Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–78.
Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, et al. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat. The role of epithelial neutrophil activating protein. J Clin Invest. 1995;95:134–41.
Caty MG, Guice KS, Oldham KT, Remick DG, Kunkel SI. Evidence for tumor necrosis factor-induced pulmonary microvascular injury after intestinal ischemia-reperfusion injury. Ann Surg. 1990;212:694–700.
Seekamp A, Mulligan MS, Till GO, Smith CW, Miyasaka M, Tamatani T, et al. Role of beta 2 integrins and ICAM-1 in lung injury following ischemia-reperfusion of rat hind limbs. Am J Pathol. 1993;143:464–72.
Dwivedi AJ, Wu R, Nguyen E, Higuchi S, Wang H, Krishnasastry K, et al. Adrenomedullin and adrenomedullin binding protein-1 prevent acute lung injury after gut ischemia-reperfusion. J Am Coll Surg. 2007;205:284–93.
Yost CS, Matthay MA, Gropper MA. Etiology of acute pulmonary edema during liver transplantation: a series of cases with analysis of the edema fluid. Chest. 2001;119:219–23.
Kostopanagiotou G, Routsi C, Smyrniotis V, Lekka ME, Kitsiouli E, Arkadopoulos N, et al. Alterations in bronchoalveolar lavage fluid during ischemia-induced acute hepatic failure in the pig. Hepatology. 2003;37:1130–8.
Colletti LM, Burtch GD, Remick DG, Kunkel SL, Strieter RM, Guice KS, et al. The production of tumor necrosis factor alpha and the development of a pulmonary capillary injury following hepatic ischemia/reperfusion. Transplantation. 1990;49:268–72.
Suzuki S, Nakamura S, Serizawa A, Sakaguchi T, Konno H, Muro H, et al. Role of Kupffer cells and the spleen in modulation of endotoxin-induced liver injury after partial hepatectomy. Hepatology. 1996;24:219–25.
Glasgow SC, Ramachandran S, Csontos KA, Jia J, Mohanakumar T, Chapman WC. Interleukin-1beta is prominent in the early pulmonary inflammatory response after hepatic injury. Surgery. 2005;138:64–70.
Alwall N, Lunderquist A, Olsson O. Studies on electrolyte-fluid retention. I. Uremic lung, fluid lung? On pathogenesis and therapy; a preliminary report. Acta Med Scand. 1953;146:157–63.
Bass HE, Singer E. Pulmonary changes in uremia. J Am Med Assoc. 1950;144:819–23.
Gibson DG. Haemodynamic factors in the development of acute pulmonary oedema in renal failure. Lancet. 1966;2:1217–20.
Acknowledgments
KD is a recipient of a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture in Japan and Takeda Science Foundation.
Conflict of interest
All the authors have declared no competing interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Doi, K., Ishizu, T., Fujita, T. et al. Lung injury following acute kidney injury: kidney–lung crosstalk. Clin Exp Nephrol 15, 464–470 (2011). https://doi.org/10.1007/s10157-011-0459-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-011-0459-4