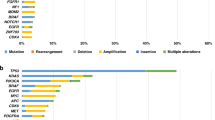
Abstract
Background
With the development of precision oncology, Molecular Tumor Boards (MTB) are developing in many institutions. However, the implementation of MTB in routine clinical practice has still not been thoroughly studied.
Material and methods
Since the first drugs approved for targeted therapies, patient tumor samples were centralized to genomic testing platforms. In our institution, all tumor samples have been analyzed since 2014 by Next Generation Sequencing (NGS). In 2015, we established a regional MTB to discuss patient cases with 1 or more alterations identified by NGS, in genes different from those related to drug approval. We conducted a retrospective comparative analysis to study whether our MTB increased the prescriptions of Molecular Targeted Therapies (MTT) and the inclusions of patients in clinical trials with MTT, in comparison with patients with available NGS data but no MTB discussion.
Results
In 2014, 86 patients had UGA, but the results were not available to clinicians and not discussed in MTB. During the years 2015 and 2016, 113 patients with an UGA (unreferenced genomic alteration) were discussed in MTB. No patients with an UGA were included in 2014 in a clinical trial, versus 2 (2%) in 2015–2016. 13 patients with an UGA (12%) were treated in 2015–2016 with a MTT whereas in 2014, no patient (p = 0.001).
Conclusions
In this retrospective analysis, we showed that the association of large-scale genomic testing and MTB was feasible, and could increase the prescription of MTT. However, in routine clinical practice, the majority of patients with UGA still do not have access to MTT.


Similar content being viewed by others
References
Hauschild A, Grob J-J, Demidov LV et al (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet Lond Engl 380(9839):358–365
Chapman PB, Hauschild A, Robert C et al (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364(26):2507–2516
Robert C, Karaszewska B, Schachter J et al (2015) Improved overall survival in melanoma with combined dabrafenib and trametinib [Internet]. N Engl J Med. https://doi.org/10.1056/NEJMoa1412690
Pagès A, Foulon S, Zou Z et al (2016) The cost of molecular-guided therapy in oncology: a prospective cost study alongside the MOSCATO trial. Genet Med 19:683–690
Massard C, Michiels S, Ferté C et al (2017) High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov 7(6):586–595
André F, Bachelot T, Commo F et al (2014) Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol 15(3):267–274
Le Tourneau C, Kamal M, Tsimberidou A-M et al (2016) Treatment algorithms based on tumor molecular profiling: the essence of precision medicine trials. J Natl Cancer Inst 108(4)
Schwaederle M, Zhao M, Lee JJ et al (2016) Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms: a meta-analysis. JAMA Oncol 2(11):1452–1459
Knepper TC, Bell GC, Hicks JK et al (2017) Key lessons learned from moffitt’s molecular tumor board: the clinical genomics action committee experience. Oncologist 22(2):144–151
Kaderbhai CG, Boidot R, Beltjens F et al (2016) Use of dedicated gene panel sequencing using next generation sequencing to improve the personalized care of lung cancer. Oncotarget 7(17):24860–24870
Harada S, Arend R, Dai Q et al (2017) Implementation and utilization of the molecular tumor board to guide precision medicine. Oncotarget 8(34):57845–57854
Beltran H, Eng K, Mosquera JM et al (2015) Whole-exome sequencing of metastatic cancer and biomarkers of treatment response. JAMA Oncol 1(4):466–474
Laskin J, Jones S, Aparicio S et al (2015) Lessons learned from the application of whole-genome analysis to the treatment of patients with advanced cancers. Cold Spring Harb Mol Case Stud 1(1):a000570
Parker BA, Schwaederlé M, Scur MD et al (2015) Breast cancer experience of the molecular tumor board at the University of California, San Diego Moores Cancer Center. J Oncol Pract 11(6):442–449
Ortiz MV, Kobos R, Walsh M et al (2016) Integrating genomics into clinical pediatric oncology using the Molecular Tumor Board at the Memorial Sloan Kettering Cancer Center. Pediatr Blood Cancer 63(8):1368–1374
Blasinska-Morawiec M, Tubiana-Mathieu N, Fougeray R et al (2013) Phase II study of intravenous vinflunine after failure of first-line vinorelbine based regimen for advanced breast cancer. Breast Edinb Scotl 22(1):58–63
Basse C, Morel C, Alt M et al (2018) Relevance of a molecular tumour board (MTB) for patients’ enrolment in clinical trials: experience of the Institut Curie. ESMO Open 3(3):e000339
Rolfo C, Manca P, Salgado R et al (2018) Multidisciplinary molecular tumour board: a tool to improve clinical practice and selection accrual for clinical trials in patients with cancer. ESMO Open [Internet]. 3(5). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6069914/. Accessed 28 Oct 2018
Liao X, Lochhead P, Nishihara R et al (2012) Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med 367(17):1596–1606
Guo J, Si L, Kong Y, Flaherty KT et al (2011) Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 29(21):2904–2909
Carvajal RD, Antonescu CR, Wolchok JD et al (2011) KIT as a therapeutic target in metastatic melanoma. JAMA 305(22):2327–2334
Tu H-Y, Ke E-E, Yang J-J et al (2017) A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer Amst Neth 114:96–102
Shi H, Moriceau G, Kong X et al (2012) Preexisting MEK1 exon 3 mutations in V600E/KBRAF melanomas do not confer resistance to BRAF inhibitors. Cancer Discov 2(5):414–424
Watson IR, Li L, Cabeceiras PK, Mahdavi M et al (2014) The RAC1 P29S hotspot mutation in melanoma confers resistance to pharmacological inhibition of RAF. Cancer Res 74(17):4845–4852
Nikolaev SI, Rimoldi D, Iseli C et al (2011) Exome sequencing identifies recurrent somatic MAP2K1 and MAP2K2 mutations in melanoma. Nat Genet 44(2):133–139
Tafe LJ, Gorlov IP, de Abreu FB et al (2015) Implementation of a Molecular Tumor Board: the impact on treatment decisions for 35 patients evaluated at Dartmouth-Hitchcock Medical Center. Oncologist 20(9):1011–1018
Trédan O, Wang Q, Pissaloux D et al (2019) Molecular screening program to select molecular-based recommended therapies for metastatic cancer patients: analysis from the ProfiLER trial. Ann Oncol Off J Eur Soc Med Oncol 30(5):757–765
Presley CJ, Tang D, Soulos PR et al (2018) Association of broad-based genomic sequencing with survival among patients with advanced non-small cell lung cancer in the community oncology setting. JAMA 320(5):469–477
Chalmers ZR, Connelly CF, Fabrizio D et al (2017) Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 9(1):34
Zehir A, Benayed R, Shah RH et al (2017) Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23(6):703–713
Nishino M, Ramaiya NH, Hatabu H et al (2017) Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 14(11):655–668
Yarchoan M, Hopkins A, Jaffee EM (2017) Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 377(25):2500–2501
Van Allen EM, Miao D, Schilling B et al (2015) Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350(6257):207–211
Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. PubMed—NCBI [Internet]. https://www-ncbi-nlm-nih-gov.passerelle.univ-rennes1.fr/pubmed/?term=Mismatch+repair+deficiency+predicts+response+of+solid+tumors+to+PD-1+blockade. Accessed 13 Oct 2018
Rizvi NA, Hellmann MD, Snyder A et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348(6230):124–128
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Bourien, H., Lespagnol, A., Campillo-Gimenez, B. et al. Implementation of a molecular tumor board at a regional level to improve access to targeted therapy. Int J Clin Oncol 25, 1234–1241 (2020). https://doi.org/10.1007/s10147-020-01661-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01661-6