Abstract
Background
Chemotherapy is one of the main treatments for lung cancer, and in these patients, discontinuation of treatment due to uncontrollable hypersensitivity reactions (HSRs) is an important problem.
Aim
To determine the frequency of HSRs during chemotherapy and to review current approaches.
Methods
We did a cross sectional study in patients undergoing chemotherapy for lung cancer in a reference chemotherapy unit from January 2012 to January 2013. Patients who developed immediate-HSRs or delayed-HSRs to chemotherapeutics and gave consent were included into study. The effectiveness of a standardised 12-step “rapid drug desensitisation” (RDD) procedure was investigated in patients with immediate-HSRs.
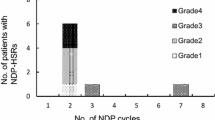
Results
In total, 1,099 cycles of chemotherapy were administered to 292 patients in 1 year. We observed ten HSRs, during ten cycles in ten patients (~3 % of the patients). Two HSRs were delayed-type, eight were immediate-type at grade 1–3. Of those with immediate-type HSR, five patients with grade 2–3, and additional two referred patients with grade 4 HSRs were successfully given their culprit drug in 35 cycles of chemotherapy with 12-step or modified 20-step RDD protocol.
Conclusions
HSRs to chemotherapeutics are not so rare. Premedication alone does not prevent such reactions. The results of RDD treatment look promising for continuing treatment with the culprit chemotherapeutic agent.

Similar content being viewed by others
Abbreviations
- HSR:
-
Hypersensitivity reaction
- SIR:
-
Standard infusion reaction
- RDD:
-
Rapid drug desensitisation
- SPT:
-
Skin prick test
References
Cernadas JR, Brockow K, Romano A et al (2010) For the European Network of Drug Allergy and the EAACI interest group on drug hypersensitivity general considerations on rapid desensitisation for drug hypersensitivity—a consensus statement. Position Paper. Allergy 65:1357–1366
Solensky R, Khan DA, Bernstein IL, Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology et al (2010) Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 105(4):259–273
Lee CW, Matulonis UA, Castells MC (2004) Carboplatin hypersensitivity: a 6-h 12-step protocol effective in 35 desensitisations in patients with gynecological malignancies and mast cell/IgE-mediated reactions. Gynecol Oncol 95:370–376
Lee CW, Matulonis UA, Castells MC (2005) Rapid inpatient/outpatient desensitisation for chemotherapy hypersensitivity: standard protocol effective in 57 patients for 255 courses. Gynecol Oncol 99:393–399
Feldweg AM, Lee CW, Matulonis UA et al (2005) Rapid desensitisation for hypersensitivity reactions to paclitaxel and docetaxel: a new standard protocol used in 77 successful treatments. Gynecol Oncol 96:824–829
Castells MC, Tennant NM, Sloane DE et al (2008) Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitisation in 413 cases. J Allergy Clin Immunol 122:574–580
The National Cancer Institute Common Toxicity Criteria (NCICTC). National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0. (CTCAE). Available at http://ctep.cancer.gov/forms/CTCAEv3.pdf. Accessed 20 July 2014
Ring J, Messmer K (1977) Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet 26:466–469
Ring J, Brockow K, Behrendt H (2004) History and classification of anaphylaxis. Novartis Found Symp 257:6–16
Goksel O, Aydın O, Atasoy C et al (2011) Hypersensitivity reactions to contrast media: prevalence, risk factors and the role of skin tests in diagnosis–a cross-sectional survey. Int Arch Allergy Immunol 155(3):297–305
Brockow K, Romano A, Blanca M et al (2002) General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy 57:45–51
Brockow K, Garvey LH, Aberer W et al (2013) ENDA/EAACI Drug Allergy Interest Group. Skin test concentrations for systemically administered drugs—an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 68(6):702–712
Wiernik PH, Schwartz EL, Strauman JJ et al (1987) Phase I clinical and pharmacokinetic study of taxol. Cancer Res 47:2486–2493
Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD et al (1994) European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. J Clin Oncol 12:2654–2666
Kwon JS, Elit L (2002) Finn Met, al. A comparison of two prophylactic regimens for hypersensitivity reactions to paclitaxel. Gynecol Oncol 84:420–425
Markman M, Kennedy A, Webster K et al (2000) Paclitaxel-associated hypersensitivity reactions: experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J Clin Oncol 18:102–105
Leguy-Seguin V, Jolimoy G, Coudert B et al (2007) Diagnostic and predictive value of skin testing in platinum salt hypersensitivity. J Allergy Clin Immunol 119:726–730
Zanotti KM, Rybicki LA, Kennedy AW et al (2001) Carboplatin skin testing: a skin-testing protocol for predicting hypersensitivity to carboplatin chemotherapy. J Clin Oncol 19:3126–3129
Markman M, Zanotti K, Peterson G et al (2003) Expanded experience with an intradermal skin test to predict for the presence or absence of carboplatin hypersensitivity. J Clin Oncol 21:4611–4614
Gadducci A, Tana R, Teti G et al (2008) Analysis of the pattern of hypersensitivity reactions in patients receiving carboplatin retreatment for recurrent ovarian cancer. Int J Gynecol Cancer 18:615–620
Markman M, Kennedy A, Webster K et al (1999) Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol 17:1141–1145
Lin RY, Schwartz LB, Curry A et al (2000) Histamine and tryptase levels in patients with acute allergic reactions: an emergency department-based study. J Allergy Clin Immunol 106:65–71
Brown SGA, Blackmean KE, Heddle RJ (2004) Can serum mast cell tryptase help diagnose anaphylaxis? Emerg Med Australas 16:120–124
Sampson HA, Muñoz-Furlong A, Campbell RL et al (2006) Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 117:373–380
Pumphrey RSH, Stanworth SJ (1996) The clinical spectrum of anaphylaxis in north-west England. Clin Exp Allergy 26:1364–1370
Treudler R, Kozovska Y, Simon JC (2008) Severe immediate-type hypersensitivity reactions in 105 German adults: when to diagnose anaphylaxis. J Investig Allergol Clin Immunol 18(1):52–58
Castells M (2009) Rapid desensitisation for hypersensitivity reactions to medications. Immunol Allergy Clin N Am 29(3):585–606
Castells M, Sancho-Serra M, Simarro M (2012) Hypersensitivity to antineoplastic agents: mechanisms and treatment with rapid desensitisation. Cancer Immunol Immunother 61:1575–1584
Acknowledgments
We would like to thank our dedicated nurses and laboratory technicians Sirin Barut, Sefika Aksoy, Sultan Ferrahoglu, Nezahat Yeni, Sebile Sezgin for their sincere assistance during RDD and SPT procedures.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Goksel, O., Goksel, T., Cok, G. et al. Hypersensitivity to chemotherapeutics: a cross sectional study with 35 desensitisations. Int J Clin Oncol 20, 395–404 (2015). https://doi.org/10.1007/s10147-014-0722-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-014-0722-2