Abstract
Objective
FOLFIRI is a standard chemotherapy regimen for the treatment of metastatic colorectal cancer. Although some studies have shown its efficacy in combination with bevacizumab as first-line chemotherapy, there are no data to support FOLFIRI plus bevacizumab as second-line chemotherapy in patients with this form of cancer. The aim of this study was to evaluate the efficacy and safety of FOLFIRI and bevacizumab as second-line chemotherapy in patients with metastatic colorectal cancer.
Methods
Eligible patients were ≥20 years old, previously treated (except with irinotecan [CPT-11] and bevacizumab), with an Eastern Cooperative Oncology Group performance status of 0, 1, or 2, and adequate organ function. Twenty-five eligible patients received FOLFIRI with bevacizumab at a dose of 10 mg/kg given intravenously on day 1. All therapy was administered every 2 weeks until disease progression. The primary endpoint was the response rate.
Results
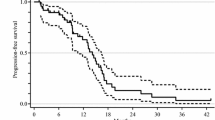
Twenty-five patients were enrolled between February 2008 and March 2009. The median age was 62 (range 38–73) years, the male/female distribution was 20/5, 16 patients had performance status 0 and 9 had performance status 1, and the proportion of patients who were oxaliplatin pretreated/untreated was 16/9. The overall response rate was 32% (90% confidence interval [CI]: 17.0–50.4%), with 8 patients showing partial responses, 15 with stable disease, and 2 with disease progression. Median progression-free survival was 11.6 months (95% CI: 6.9–16.4). Median overall survival was 21.4 months (95% CI: 12.0–30.8). The grade 3/4 adverse events with treatment were neutropenia (64%), leukopenia (16%), diarrhea (8%), anorexia (8%), and febrile neutropenia (8%). The bevacizumab-related grade 3/4 adverse event was hypertension, which was observed in 12% of patients.
Conclusions
The FOLFIRI plus bevacizumab regimen is an active, well-tolerated second-line chemotherapy treatment for patients with metastatic colorectal cancer.

Similar content being viewed by others
References
Jemal A, Tiwari RC, Murray T et al (2004) American Cancer Society, Cancer Statistics 2004. CA Cancer J Clin 54:8–29
Parkin DM (2001) Global cancer statistics in the year 2000. Lancet Oncol 2:533–543
Cancer Statistics in Japan Editorial Board. Cancer Statistics in Japan 2008. Foundation for Promotion of Cancer Research 2008 (in Japanese)
Tsukuma H, Ajiki W, Ioka A et al (2006) Survival of cancer patients diagnosed between 1993 and 1996: a collaborative study of population-based cancer registries in Japan. Jpn J Clin Oncol 36:602–607
Douillard JY, Cunningham D, Roth AD et al (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet 355:1041–1047
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342
Fuchs CS, Marshall J, Barrueco J (2008) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol 26(4):689–690
NCCN Clinical Practice Guidelines. http://www.nccn.org/index.asp
Therasse P, Arbuck S, Eisenhauer E et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
National Cancer Institute—Common Toxicity Criteria (NCI-CTC Version 3.0, March 31, 2003). http://www.jcog.jp
Giantonio BJ, Catalano PJ, Meropol NJ et al (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25:1539–1544
Tournigand C, Andre T, Achille E et al (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237
Muro K, Boku N, Shimada Y et al (2010) Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomized phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol 11:853–860
Padera TP, Stoll BR, Tooredman JB et al (2004) Pathology: cancer cells compress intratumour vessels. Nature 427(6976):695
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307(5706):58–62
Acknowledgments
We would like to thank Ms. Hideko Morita and Ms. Makiko Shinogi for their help in collecting and organizing the clinical samples, and Ms. Michiyo Tada and Mr Hiroshi Nagai for their help in data management for this study.
Conflict of interest
No author has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Horita, Y., Yamada, Y., Kato, K. et al. Phase II clinical trial of second-line FOLFIRI plus bevacizumab for patients with metastatic colorectal cancer: AVASIRI trial. Int J Clin Oncol 17, 604–609 (2012). https://doi.org/10.1007/s10147-011-0331-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-011-0331-2