Abstract
Intradural spinal tumors present significant challenges due to involvement of critical motor and sensory tracts. Achieving maximal resection while preserving functional tissue is therefore crucial. Fluorescence-guided surgery aims to improve resection accuracy and is well studied for brain tumors, but its efficacy has not been fully assessed for spinal tumors. This meta-analysis aims to delineate the efficacy of fluorescence guidance in intradural spinal tumor resection. The authors performed a systematic review in four databases. We included studies that have utilized fluorescence agents, 5-aminolevulinic acid (5-ALA) or sodium fluorescein, for the resection of intradural spinal tumors. A meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. A total of 12 studies involving 552 patients undergoing fluorescence-guided intradural spinal tumor resection were included. Meningiomas demonstrated a 98% fluorescence rate and were associated with a homogenous florescence pattern; however, astrocytomas had variable fluorescence rate with pooled proportion of 70%. There was no significant difference in gross total resection (GTR) rates between fluorescein and 5-ALA (94% vs 84%, p = .22). Pre-operative contrast enhancement was significantly associated with intraoperative fluorescence with fluorescein. Intramedullary tumors with positive intraoperative fluorescence were significantly associated with higher GTR rates (96% vs 73%, p = .03). Utilizing fluorescence guidance during intradural spinal tumor resection holds promise of improving intraoperative visualization for specific intradural spinal tumors. Meningiomas and ependymomas have the highest fluorescence rates especially with sodium fluorescein; on the other hand, astrocytomas have variable fluorescence rates with no superiority of either agent. Positive fluorescence of intramedullary tumors is associated with a higher degree of resection.





Similar content being viewed by others
Data availability
Data and materials used in this systematic review are available upon request.
References
Garcés-Ambrossi GL, McGirt MJ, Mehta VA et al (2009) Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine 11:591–599. https://doi.org/10.3171/2009.4.SPINE08159
Wostrack M, Ringel F, Eicker SO et al (2018) Spinal ependymoma in adults: a multicenter investigation of surgical outcome and progression-free survival. J Neurosurg Spine 28:654–662. https://doi.org/10.3171/2017.9.SPINE17494
Tarapore PE, Modera P, Naujokas A et al (2013) Pathology of spinal ependymomas: an institutional experience over 25 years in 134 patients. Neurosurgery 73:247–255. https://doi.org/10.1227/01.neu.0000430764.02973.78
Acerbi F, Broggi M, Eoli M et al (2014) Is fluorescein-guided technique able to help in resection of high-grade gliomas? Neurosurg Focus 36:E5. https://doi.org/10.3171/2013.11.FOCUS13487
Acerbi F, Broggi M, Schebesch K-M et al (2018) Fluorescein-guided surgery for resection of high-grade gliomas: a multicentric prospective phase II study (FLUOGLIO). Clin Cancer Res Off J Am Assoc Cancer Res 24:52–61. https://doi.org/10.1158/1078-0432.CCR-17-1184
Bowden SG, Neira JA, Gill BJA et al (2018) Sodium fluorescein facilitates guided sampling of diagnostic tumor tissue in nonenhancing gliomas. Neurosurgery 82:719. https://doi.org/10.1093/neuros/nyx271
Hadjipanayis CG, Widhalm G, Stummer W (2015) What is the surgical benefit of utilizing 5-ALA for fluorescence-guided surgery of malignant gliomas? Neurosurgery 77:663–673. https://doi.org/10.1227/NEU.0000000000000929
Nagaya T, Nakamura YA, Choyke PL, Kobayashi H (2017) Fluorescence-guided surgery. Front. Oncol 7:314. https://doi.org/10.3389/fonc.2017.00314
Puppa AD, Ciccarino P, Lombardi G et al (2014) 5-Aminolevulinic acid fluorescence in high grade glioma surgery: surgical outcome, Intraoperative Findings, and Fluorescence Patterns. BioMed Res Int 2014:e232561. https://doi.org/10.1155/2014/232561
Muto J, Mine Y, Nagai S et al (2022) Utility of intraoperative real-time near-infrared fluorescence surgery for spinal schwannoma. Neurosurg Focus Video 6:V12. https://doi.org/10.3171/2021.10.FOCVID21158
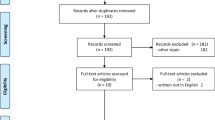
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
McCormack T, Karaikovic E, Gaines RW (1994) The load sharing classification of spine fractures. Spine 19:1741–1744. https://doi.org/10.1097/00007632-199408000-00014
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605. https://doi.org/10.1007/s10654-010-9491-z
Sterne JA, Egger M (2001) Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54:1046–1055. https://doi.org/10.1016/s0895-4356(01)00377-8
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor Package. J Stat Softw 36:1–48. https://doi.org/10.18637/jss.v036.i03
Viechtbauer W (2010) Conducting Meta-Analyses in R with themetafor Package. J Stat Softw 36:1–48. https://doi.org/10.18637/jss.v036.i03
Lipsey MW, Wilson DB (2001) Practical meta-analysis. Sage Publications, Inc, Thousand Oaks, CA, US
McHugh ML (2013) The Chi-square test of independence. Biochem Medica 23:143–149. https://doi.org/10.11613/BM.2013.018
Millesi M, Kiesel B, Mazanec V et al (2020) 5-ALA fluorescence for intraoperative visualization of spinal ependymal tumors and identification of unexpected residual tumor tissue: experience in 31 patients. J Neurosurg Spine 34:374–382. https://doi.org/10.3171/2020.6.SPINE20506
Inoue T, Endo T, Nagamatsu K et al (2013) 5-aminolevulinic acid fluorescence-guided resection of intramedullary ependymoma: report of 9 cases. Neurosurgery 72:159–168. https://doi.org/10.1227/NEU.0b013e31827bc7a3
Millesi M, Kiesel B, Woehrer A et al (2014) Analysis of 5-aminolevulinic acid-induced fluorescence in 55 different spinal tumors. Neurosurg Focus 36:E11. https://doi.org/10.3171/2013.12.FOCUS13485
Ung TH, Serva S, Chatain GP et al (2022) Application of sodium fluorescein for spinal cord lesions: intraoperative localization for tissue biopsy and surgical resection. Neurosurg Rev 45:1563–1569. https://doi.org/10.1007/s10143-021-01676-1
Acerbi F, Cavallo C, Schebesch K-M et al (2017) Fluorescein-guided resection of intramedullary spinal cord tumors: results from a preliminary, multicentric, retrospective study. World Neurosurg 108:603–609. https://doi.org/10.1016/j.wneu.2017.09.061
Cardali SM, Ricciardo G, Garufi G et al (2022) Fluorescein-guided surgery for intradural spinal tumors: a single-center experience. Brain Spine 2:100908. https://doi.org/10.1016/j.bas.2022.100908
Sun Z, Jing L, Fan Y et al (2020) Fluorescein-guided surgery for spinal gliomas: analysis of 220 consecutive cases. Int Rev Neurobiol 151:139–154. https://doi.org/10.1016/bs.irn.2020.03.004
Goryaynov SA, Okhlopkov VA, Golbin DA et al (2019) Fluorescence diagnosis in neurooncology: retrospective analysis of 653 cases. Front Oncol 9:830. https://doi.org/10.3389/fonc.2019.00830
Moreno RG, García LMB, Bastidas HI et al (2019) Fluorescence guided surgery with 5-aminolevulinic acid for resection of spinal cord ependymomas. Asian Spine J 13:119–125. https://doi.org/10.31616/asj.2018.0165
Sun Z, Yuan D, Sun Y et al (2022) Intraoperative application of yellow fluorescence in resection of intramedullary spinal canal ependymoma. J Int Med Res 50:3000605221082889. https://doi.org/10.1177/03000605221082889
Olguner SK, Arslan A, Açık V et al (2020) Sodium fluorescein for spinal intradural tumors. Front Oncol 10:618579. https://doi.org/10.3389/fonc.2020.618579
Eicker SO, Floeth FW, Kamp M et al (2013) The impact of fluorescence guidance on spinal intradural tumour surgery. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 22:1394–1401. https://doi.org/10.1007/s00586-013-2657-0
Stummer W, Pichlmeier U, Meinel T et al (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401. https://doi.org/10.1016/S1470-2045(06)70665-9
Chen B, Wang H, Ge P et al (2012) Gross total resection of glioma with the intraoperative fluorescence-guidance of fluorescein Sodium. Int J Med Sci 9:708–714. https://doi.org/10.7150/ijms.4843
Dilek O, Ihsan A, Tulay H (2011) Anaphylactic reaction after fluorescein sodium administration during intracranial surgery. J Clin Neurosci 18:430–431. https://doi.org/10.1016/j.jocn.2010.06.012
Tanahashi S, Lida H, Dohi S (2006) An anaphylactoid reaction after administration of fluorescein sodium during neurosurgery. Anesth Analg 103:503. https://doi.org/10.1213/01.ANE.0000227205.37935.10
Chung IWH, Eljamel S (2013) Risk factors for developing oral 5-aminolevulinic acid-induced side effects in patients undergoing fluorescence guided resection. Photodiagnosis Photodyn Ther 10:362–367. https://doi.org/10.1016/j.pdpdt.2013.03.007
Raco A, Esposito V, Lenzi J et al (2005) Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery 56:972. https://doi.org/10.1227/01.NEU.0000158318.66568.CC
Hongo H, Takai K, Komori T, Taniguchi M (2018) Intramedullary spinal cord ependymoma and astrocytoma: intraoperative frozen-section diagnosis, extent of resection, and outcomes. J Neurosurg Spine 30:133–139. https://doi.org/10.3171/2018.7.SPINE18230
Bakhshi SK, Waqas M, Shakaib B, Enam SA (2016) Management and outcomes of intramedullary spinal cord tumors: a single center experience from a developing country. Surg Neurol Int 7:S617–S622. https://doi.org/10.4103/2152-7806.189733
Rashad S, Elwany A, Farhoud A (2018) Surgery for spinal intramedullary tumors: technique, outcome and factors affecting resectability. Neurosurg Rev 41:503–511. https://doi.org/10.1007/s10143-017-0879-z
Li T-Y, Chu J-S, Xu Y-L et al (2014) Surgical strategies and outcomes of spinal ependymomas of different lengths: analysis of 210 patients: clinical article. J Neurosurg Spine 21:249–259. https://doi.org/10.3171/2014.3.SPINE13481
Azad TD, Pendharkar AV, Pan J et al (2018) Surgical outcomes of pediatric spinal cord astrocytomas: systematic review and meta-analysis. J Neurosurg Pediatr 22:404–410. https://doi.org/10.3171/2018.4.PEDS17587
Hersh AM, Patel J, Pennington Z et al (2022) Perioperative outcomes and survival after surgery for intramedullary spinal cord tumors: a single-institution series of 302 patients. J Neurosurg Spine 37:252–262. https://doi.org/10.3171/2022.1.SPINE211235
Schupper AJ, Rao M, Mohammadi N et al (2021) Fluorescence-Guided Surgery: A Review on Timing and Use in Brain Tumor Surgery. Front Neurol 12:682151. https://doi.org/10.3389/fneur.2021.682151
Schebesch K-M, Proescholdt M, Höhne J et al (2013) Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery—a feasibility study. Acta Neurochir (Wien) 155:693–699. https://doi.org/10.1007/s00701-013-1643-y
Shinoda J, Yano H, Yoshimura S-I et al (2003) Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Technical note. J Neurosurg 99:597–603. https://doi.org/10.3171/jns.2003.99.3.0597
Palasuberniam P, Kraus D, Mansi M et al (2019) Ferrochelatase deficiency abrogated the enhancement of aminolevulinic acid-mediated protoporphyrin IX by iron chelator deferoxamine. Photochem Photobiol 95:1052–1059. https://doi.org/10.1111/php.13091
Fontana AO, Piffaretti D, Marchi F et al (2017) Epithelial growth factor receptor expression influences 5-ALA induced glioblastoma fluorescence. J Neurooncol 133:497–507. https://doi.org/10.1007/s11060-017-2474-0
Lau D, Hervey-Jumper SL, Chang S et al (2016) A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J Neurosurg 124:1300–1309. https://doi.org/10.3171/2015.5.JNS1577
Stummer W, Rodrigues F, Schucht P et al (2014) Predicting the “usefulness” of 5-ALA-derived tumor fluorescence for fluorescence-guided resections in pediatric brain tumors: a European survey. Acta Neurochir (Wien) 156:2315. https://doi.org/10.1007/s00701-014-2234-2
Elliott JT, Dsouza AV, Davis SC et al (2015) Review of fluorescence guided surgery visualization and overlay techniques. Biomed Opt Express 6:3765–3782. https://doi.org/10.1364/BOE.6.003765
Mischkulnig M, Traxler D, Wadiura LI et al (2023) Comparison of minimal detectable protoporphyrin IX concentrations with a loupe device and conventional 5-ALA fluorescence microscopy: an experimental study. J Biomed Opt 28:106004. https://doi.org/10.1117/1.JBO.28.10.106004
Author information
Authors and Affiliations
Contributions
HA, CH, PG, PZ, RF, IA: conception and design, provision of study patient, data analysis and interpretation, manuscript writing, final approval of the manuscript. AA, OB, IA, AS: collection and assembly of data, data analysis and interpretation, manuscript writing. All authors: writing the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information

Supplementary Figure 1
Newcastle-Ottawa scale for non-randomized studies to determine the quality of the studies

Supplementary Figure 2
Funnel plots for determining the risk of publication bias.

Supplementary Figure 3
Forest plots showing pooled proportions of fluorescence with subgroup analysis based on the administered agent for ependymomas.

Supplementary Figure 4
Forest plots showing pooled proportions of fluorescence with subgroup analysis based on the administered agent for astrocytomas.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Albalkhi, I., Shafqat, A., Bin-Alamer, O. et al. Fluorescence-guided resection of intradural spinal tumors: a systematic review and meta-analysis. Neurosurg Rev 47, 10 (2024). https://doi.org/10.1007/s10143-023-02230-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02230-x