Abstract
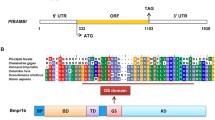
The BMP2 signal transduced by SMAD1/5 plays an important role in osteoblast differentiation and bone formation. Shell formation of Pinctada fucata martensii is a typical biomineralization process that is similar to that of teeth/bone formation. However, whether the Pinctada fucata BMP2 (PfBMP2) signal transduced by PfSMAD1/5 occurs in P. f. martensii, how the PfBMP2 signal is transduced by PfSMAD1/5, and how PfSMAD1/5 regulates the biomineralization process in this species and other shellfish are poorly understood. Therefore, injection experiments of recombinant PfBMP2 and inhibitor dorsomorphin revealed that PfSMAD1/5 can transduce PfBMP2 signals. Subcellular localization and bimolecular fluorescence complementation assays indicated that PfSMAD1/5 phosphorylated by PfBMPR1b interacts with PfSMAD4 in the cytoplasm to form a complex, which translocates to the nucleus to transduce PfBMP2 signals. Co-immunoprecipitation and luciferase assays revealed that PfSMAD1/5 may interact with PfMSX to dislodge it from its binding element, resulting in initiation of mantle gene transcription. The in vivo functional assay showed that knockdown of PfMSAD1/5 decreased expression of shell matrix genes and disordered the nacreous layer, and the correlation assay of shell regeneration showed the concomitant expression pattern of PfSMAD1/5 and shell matrix genes. Together, these data showed that PfSMAD1/5 can transduce PfBMP2 signals to regulate shell biomineralization in P. f. martensii, which illustrated conservation of the BMP2-SMAD signal pathway among invertebrates. Particularly, the results suggest that there is only one PfMSX gene, which functions like the Hox gene in vertebrates, that interacts with PfSMAD1/5 in a protein–protein action form and plays the role of transcription repressor.







Similar content being viewed by others
Abbreviations
- TGF:
-
Transcription growth factor
- BMP:
-
Bone morphogenetic protein
- BMPR:
-
BMP receptor
- HOX:
-
Homotypic box gene
- IPTG:
-
Isopropyl β-d-1-thiogalactopyranoside
- SDS-PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- CO-IP:
-
Co-immunoprecipitation
- RT-PCR:
-
Real-time polymerase chain reaction
- BSA:
-
Bovine serum albumin
- FITC:
-
Fluorescein isothiocyanate
- DAPI:
-
6-Diamidino-2-pheny-lindole
- BiFC:
-
Bimolecular fluorescence complementation
- WT:
-
Wild type
- MU:
-
Mutation
- RNAi:
-
RNA interference
- SEM:
-
Scanning electron microscopy
- SBE:
-
SMAD binding element
- Bambi:
-
BMP and activin membrane-bound inhibitor
- EYFP:
-
Enhanced yellow fluorescent protein
References
Bahn SY, Jo BH, Choi YS, Cha HJ (2017) Control of nacre biomineralization by Pif80 in pearl oyster. Sci Adv 3:e1700765
Benjamin M et al (2012) Different secretory repertoires control the biomineralization processes of prism and nacre deposition of the pearl oyster shell. Proc Natl Acad Sci U S A 109:20986–20991
Binato R, Martinez CEA, Pizzatti L, Robert B, Abdelhay E (2006) SMAD 8 binding to mice Msx1 basal promoter is required for transcriptional activation. Biochem J 393:141–150
Boergermann JH, Kopf J, Yu PB, Knaus P (2010) Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int J Biochem Cell Biol 42:1802–1807
Cao X, Chen D (2005) The BMP signaling and in vivo bone formation. Gene 357:1–8
Daisuke F et al (2014) Novel genes participating in the formation of prismatic and nacreous layers in the pearl oyster as revealed by their tissue distribution and RNA interference knockdown. Plos One 9:e84706
Fang D, Xu GR, Hu YL, Pan C, Xie LP, Zhang RQ (2011) Identification of genes directly involved in shell formation and their functions in pearl oyster, Pinctada fucata. Plos One 6:e21860
Feng D, Li Q, Yu H (2019) RNA interference by ingested dsRNA-expressing bacteria to study shell biosynthesis and pigmentation in Crassostrea gigas. Mar Biotechnol (NY) 21:526–536
Heldin CH, Miyazono K, Ten DP (1997) TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390:465–471
Herpin A, Lelong C, Becker T, Rosa F, Favrel P, Cunningham C (2010) Structural and functional evidence for a singular repertoire of BMP receptor signal transducing proteins in the lophotrochozoan Crassostrea gigas suggests a shared ancestral BMP/activin pathway. Febs J 272:3424–3440
Hu CD, Chinenov Y, Kerppola TK (2002) Visualization of interactions among bZip and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell 9:789–798
Iijima M, Takeuchi T, Sarashina I, Endo K (2008) Expression patterns of engrailed and dpp in the gastropod Lymnaea stagnalis. Dev Genes Evol 218:237–251
Jin C, Zhao JY, Liu XJ, Li JL (2019) Expressions of shell matrix protein genes in the pearl sac and its correlation with pearl weight in the first 6 months of pearl formation in Hyriopsis cumingii. Mar Biotechnol (NY) 21:240–249
Katagiri T, Osawa K, Tsukamoto S, Mai F, Miyamoto A, Mizuta T (2015) Bone morphogenetic protein-induced heterotopic bone formation: what have we learned from the history of a half century? Jpn Dent Sci Rev 51:42–50
Kim J, Johnson K, Chen HJ, Carroll S, Laughon A (1997) Drosophila Mad binds to DNA and directly mediates activation of vestigial by Decapentaplegic. Nature 388:304–308
Kin K, Kakoi S, Wada H (2009) A novel role for dpp in the shaping of bivalve shells revealed in a conserved molluscan developmental program. Dev Biol 329:152–166
Kodama Y, Hu CD (2010) An improved bimolecular fluorescence complementation assay with a high signal-to-noise ratio. Biotechniques 49:793–805
Kofron MD, Li X, Laurencin CT (2004) Protein- and gene-based tissue engineering in bone repair. Curr Opin Biotechnol 15:399–405
Lelong C, Badariotti F, Quéré HL, Rodet F, Dubos MP, Favrel P (2007) Cg-TGF-beta, a TGF-beta/activin homologue in the Pacific oyster Crassostrea gigas, is involved in immunity against gram-negative microbial infection. Dev Comp Immunol 31:30–38
Li S, Liu Y, Huang J, Zhan A, Xie L, Zhang R (2017a) The receptor genes PfBMPR1B and PfBAMBI are involved in regulating shell biomineralization in the pearl oyster Pinctada fucata. Sci Rep 7:9219
Li H, Zhang B, Fan S, Liu B, Su J, Yu D (2017b) Identification and differential expression of biomineralization genes in the mantle of pearl oyster Pinctada fucata. Mar Biotechnol (NY) 219:266–276
Liu G, Huan P, Liu BZ (2014) Cloning and expression patterns of two Smad genes during embryonic development and shell formation of the Pacific oyster Crassostrea gigas. Chin J Oceanol Limnol 32:1224–1231
Matsubara T, Kida K, Yamaguchi A, Hata K, Ichida F, Meguro H, Aburatani H, Nishimura R, Yoneda T (2008) BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J Biol Chem 283:29119–29125
Mi Z, Maoxian H, Xiande H, Qi W (2014) A homeodomain transcription factor gene, PfMSX, activates expression of Pif gene in the pearl oyster Pinctada fucata. Plos One 9:e103830
Mi Z, Yu S, He M, Huang X, Qi W (2016) PfSMAD4 plays a role in biomineralization and can transduce bone morphogenetic protein-2 signals in the pearl oyster Pinctada fucata. BMC Dev Biol 16:1–9
Miyashita T, Hanashita T, Toriyama M, Takagi R, Akashika T, Higashikubo N (2008) Gene cloning and biochemical characterization of the BMP-2 of Pinctada fucata. Biosci Biotechnol Biochem 72:37–47
Mount AS, Wheeler AP, Paradkar RP, Snider D (2004) Hemocyte-mediated shell mineralization in the eastern oyster. Science 304:297–300
Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A (2002) A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20:87–90
Nederbragt AJ, Loon AEV, Dictus WJAG (2002) Expression of Patella vulgata orthologs of engrailed and dpp-BMP2/4 in adjacent domains during molluscan shell development suggests a conserved compartment boundary mechanism. Dev Biol 246:341–355
Nishimura R, ., Kato Y, ., Chen D, ., Harris SE, Mundy GR, Yoneda T, . (1998) Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem 273:1872–1879
Onichtchouk D, Chen YG, Dosch R, Gawantka V, Dellus H, Massague J, Niehrs C (1999) Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature 401:480–485
Quéré HL, Herpin A, Huvet A, Lelong C, Favrel P (2009) Structural and functional characterizations of an Activin type II receptor orthologue from the pacific oyster Crassostrea gigas. Gene 436:101–107
Raftery LA, Sutherland DJ (1999) TGF-beta family signal transduction in Drosophila development: from Mad to Smads. Dev Biol 210:251–268
Reinhardt B, Broun M, Blitz IL, Bode HR (2004) HyBMP5-8b, a BMP5-8 orthologue, acts during axial patterning and tentacle formation in hydra. Dev Biol 267:43–59
Savage C, Das P, Finelli AL, Townsend SR, Sun CY, Baird SE, Padgett RW (1996) Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor beta pathway components. Proc Natl Acad Sci U S A 93:790–794
Shi X, Yang X, Chen D, Chang Z, Cao X (1999) Smad1 interacts with homeobox DNA-binding proteins in bone morphogenetic protein signaling. J Biol Chem 274:13711–13717
Shi Y, Xu M, Huang J, Zhang H, Liu W, Ou Z, He M (2019) Transcriptome analysis of mantle tissues reveals potential biomineralization-related genes in Tectus pyramis Born. Comp Biochem Physiol Part D Genomics Proteomics 29:131–144
Shimizu K, Sarashina I, Kagi H, Endo K (2011) Possible functions of Dpp in gastropod shell formation and shell coiling. Dev Genes Evol 221:59–68
Sierra MI, Wright MH, Nash PD (2010) AMSH interacts with ESCRT-0 to regulate the stability and trafficking of CXCR4. J Biol Chem 285:13990–14004
Suzuki M, Nagasawa H (2013) Mollusk shell structures and their formation mechanism. Can J Zool 91:349–366
Suzuki M, Saruwatari K, Kogure T, Yamamoto Y, Nishimura T, Kato T, Nagasawa H (2009) An acidic matrix protein, Pif, is a key macromolecule for nacre formation. Science 325:1388–1390
Takahashi H, Kamiya A, Ishiguro A, Suzuki AC, Saitou N, Toyoda A, Aruga J (2008) Conservation and diversification of Msx protein in metazoan evolution. Mol Biol Evol 25:69–82
Takami A, Kato H, Takagi R, Miyashita T (2013) Studies on the Pinctada fucata BMP-2 gene: structural similarity and functional conservation of its osteogenic potential within the animal kingdom. Int J Zool 2013:1–9
Vidi PA, Ejendal KF, Przybyla JA, Watts VJ (2011) Fluorescent protein complementation assays: new tools to study G protein-coupled receptor oligomerization and GPCR-mediated signaling. Mol Cell Endocrinol 331:185–193
von Bubnoff A, Cho KW (2001) Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev Biol 239:1–14
Yamamoto N, Akiyama S, Katagiri T, Namiki M, Kurokawa T, Suda T (1997) Smad1 and Smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts. Biochem Biophys Res Commun 238:574
Yan F, Luo S, Jiao Y, Deng Y, du X, Huang R, Wang Q, Chen W (2014) Molecular characterization of the BMP7 gene and its potential role in shell formation in Pinctada martensii. Int J Mol Sci 15:21215–21228
Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM (2005) Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci U S A 102:5062–5067
Yu PB et al (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4:33–41
Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE (1998) Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell 1:611–617
Zhang C, Zhang RQ (2006) Matrix proteins in the outer shells of molluscs. Mar Biotechnol 8:572–586
Zhang N, Gong ZZ, Minden M, Lu M (1993) The HOX-11 (TCL-3) homeobox proto-oncogene encodes a nuclear protein that undergoes cell cycle-dependent regulation. Oncogene 8:3265
Zhang Y, Feng X, We R, Derynck R (1996) Receptor-associated Mad homologues synergize as effectors of the TGF-beta response. Nature 383:168
Zhou YJ et al (2010) Correlations among mRNA expression levels of engrailed, BMP2 and Smad3 in mantle cells of pearl oyster Pinctada fucata. Prog Biochem Biophys 37:737–746
Zoccola D, Moya A, Beranger GE, Tambutte E, Allemand D, Carle GF, Tambutte S (2009) Specific expression of BMP2/4 ortholog in biomineralizing tissues of corals and action on mouse BMP receptor. Mar Biotechnol (NY) 11:260–269
Acknowledgments
This project was supported by the National Natural Science Foundation of China (grant no. 41606151), Natural Science Foundation of Guangdong Province, China (grant no. 2019A1515011968), the Earmarked Fund for the Modern Agro-industry Technology Research System (grant no. CARS-49), and the Science and Technology Planning Project of Guangdong Province, China (2017B0303014052).
Author information
Authors and Affiliations
Contributions
Y.S. and M.H. planned and designed the research. Y.S. performed experiments, analyzed data, and wrote the paper. M.Z. performed the subcellular localization and BiFC assay. All the authors reviewed, edited, and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, Y., Zhao, M. & He, M. PfSMAD1/5 Can Interact with PfSMAD4 to Inhibit PfMSX to Regulate Shell Biomineralization in Pinctada fucata martensii. Mar Biotechnol 22, 246–262 (2020). https://doi.org/10.1007/s10126-020-09948-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-020-09948-5