Abstract
Chlamydia trachomatis is considered as a public health problem due to its high prevalence and increased rates of gynecological disorders. The major outer membrane protein (MOMP) of this bacterium is the most abundant protein in its membrane and has been evaluated not only as a vaccine development candidate but also is used in many diagnostic tests. The MOMP weighs 69 kDa and contains four variable segments (VS 1–4) separated by constant regions. Several research groups have developed recombinant single-variable segments of MOMP expressed in Escherichia coli cytoplasm. But, all variable segments have been used minimally for the diagnosis of a chlamydial infection. In this experiment, the authors obtained the recombinant MOMP of C. trachomatis (rMOMP) in E. coli rMOMP and extracted, purified, and partially characterized it. This was later used to identify anti-Chlamydia trachomatis antibodies in sera of infertile patients by immunodetection assays, enzyme-linked immunosorbent assay (ELISA), and indirect immunofluorescence tests. The ELISA test showed high sensitivity and low specificity of 100 and 58.3%, respectively. The above results obtained were linked to the cross-reactivity of antibodies against C. pneumoniae or C. psittaci. Hence, an evaluation was performed to obtain an optimized test for the diagnosis of C. trachomatis infection.
Similar content being viewed by others
Introduction
Chlamydia trachomatis is considered as a public health problem due to its high prevalence and increased rates of gynecological disorders that are caused by a sexually transmitted infection (O’Connell and Ferone 2016). This bacterium is an obligate intracellular parasite that is divided into 19 serological variants based on the antigenicity of the major outer membrane protein (MOMP). Serovars D–K and L1–L3 strains of C. trachomatis are seen to cause urogenital tract infections, cervicitis, endometritis, and salpingitis in women. Chronic infections like pelvic inflammatory disease, ectopic pregnancy, blocked fallopian tube, and infertility are also seen to occur with serovars D–K strains (Bastidas et al. 2013). The serovars L1–L3 are also seen to cause an invasive disease called as the lymphogranuloma venereum (O’Connell and Ferone 2016; Bastidas et al. 2013).
MOMP is a species-specific membrane protein that is highly immunogenic. Hence, this membrane protein is considered as the best antigen to develop a vaccine but is also used in infection diagnosis (Wen et al. 2016). At present, several publications have informed about vaccine results in animal models, as well as the enzyme-linked immunosorbent assay (ELISA) test development with recombinant MOMP obtained from Escherichia coli cloned with expression vectors (Jiang et al. 2017; Bandehpour et al. 2006).
The gene encoding MOMP is ompA, which is one of the most polymorphic genes that is present in this bacterium and has a molecular size of 1200 bp, approximately. It shows four variables and five constant regions (Findlay et al. 2005; De Haro-Cruz et al. 2011). Mygind et al. (2000) reported that when the three-recombinant variable segments (VS1, VS2, and VS4) were used as antigens, the ELISA test showed a sensitivity of 80% and a specificity of 90%. Even more, the VS3 segment was considered an immunodominant peptide that was seen to promote and generate host protective antibodies against infection (Ishizaki et al. 1992; Batteiger et al. 1996). These results showed that MOMP recombinant poly-antigens could become a valuable tool for the serological diagnosis of Chlamydia infection (Mygind et al. 2000). The purpose of this study was to obtain an ELISA test using the partial recombinant protein of MOMP (including the four VS and excluding only the constant regions 1 and 5 to try to make the ELISA technique more sensitive and eliminate the cross-reaction with antibodies anti-C. pneumoniae) as antigen which was to be used as a serological diagnostic test of Chlamydia infection.
Material and methods
Cloning of recombinant Chlamydia MOMP
It is known that MOMP has five constant and four variable regions that are found on the surface of the outer membrane, wherein the VS1, VS2, and VS4 regions are seen to stimulate the humoral response and the VS3 region stimulates the cellular response. Due to this, our experiment mainly looked for the expression of a recombinant protein with four variable regions that allowed to recognize antibodies against C. trachomatis with a serotype or serogroup specificity.
The ompA gene from the serovar L2 strain of C. trachomatis (American Type Culture Collection (ATCC), VR-902B; GenBank accession number: KP120855.1) was amplified. An in-house polymerase chain reaction (PCR) was carried out for the amplification of an 879-bp fragment (Yang et al. 1993; De Haro-Cruz et al. 2011). P3:5′TGACTTTGTTTTCGACCGTGTTTT3′ and P4:5′TTTTCTTAAGATTTCATCTTGTTCAT/CTG3′ primers were used. PCR cycling conditions were as follows: 5 min for pre-denaturation at 95 °C, followed by 35 cycles at 95 °C/1.5 min, 60 °C/2.5 min, and 72 °C/3 min, respectively, and 72 °C/10 min for the final extension. The PCR product (879 bp) was later visualized with GelStarTM nucleic acid gel stain (Lonza, Walkersville, MD, USA) under a UV transilluminator (MultiImagenTM light cabinet, Alpha Innotech Co., San Jose, CA, USA). The ompA amplicon was directly ligated with a pGEM-T easy vector (TA cloning vector; Promega, Madison, WI, USA) and was transferred on to E. coli JM109 and was later grown on Lysogeny broth (LB) agar plates with 10 μg/mL ampicillin. Using the P3 and P4 primers, the insert containing the ompA gene was sequenced at the Cellular Physiology Department of the National Autonomous University of Mexico (UNAM), Mexico City, Mexico.
Once it was determined that the sequence corresponds to the strain of the lymphogranuloma venereum, a second amplification was performed with the primers PG1:5′ACGCGTCGCGTGACTTTGTTTTCGACCGTG3′ and PG2:5′ATAGTTTAGCGGCCGCTCATTTTCTAGATTTCATC3′ with the same reaction conditions described above. The amplified fragment was inserted into the plasmid PGEX-6P1 by cutting with the restriction enzymes Sal I and NotI construct called ompLGV. The design of these primers allows to have a transcription initiation site without stopping codon which allows the recombinant protein to have glutathione-S-transferase (GST) tails which will allow the purification of the peptide.
Protein expression and purification of the recombinant MOMP
For protein expression, E. coli strain BL21was transformed with the ompLGV construct following manufacturer’s instructions (GE Healthcare Bio-Sciences Corp. Piscataway, NJ, USA). A 5-mL aliquot of an overnight culture was sub-cultured into 800 mL fresh Luria-Bertani (LB) medium (Sigma-Aldrich, St. Louis, MO, USA) containing ampicillin (50 mg/L). Transformed cells were incubated at 37 °C to an optical density (OD; 600 nm) of 0.5–0.8 and then maintained at room temperature for 5 min. Protein expression was induced with 0.4 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) for 4 h at 37 °C, under continuous stirring at 200 rpm. The cells were harvested by centrifugation at 3500g for 15 min and resuspended in phosphate-buffered saline (PBS). Subsequently, the cells were characterized by immunofluorescence with monoclonal antibodies to the species-specific MOMP. Later, bacteria were broken two times by sonication for 15 s at 70% amplitude in pulse-mode in a Sonifier B-15P (Branson Sonic Power Co., Danbury, CT, USA).
The lysate was centrifuged at 23,000g for 40 min at 4 °C. The supernatant was filtered through a 0.45-μm pore-size filter (Millipore, Bedford, MA, USA). The GST-tagged protein was purified using glutathione sepharose beads (GE Healthcare) according to manufacturer’s instructions. The purity of the recombinant protein was verified by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and was subsequently concentrated to a final concentration of 1 mg/L using a 10-kDa cutoff Amicon protein concentrator (YM-10; Millipore, Corporation, Bedford, MA, USA). The final protein concentration was determined by the Bradford assay (Bradford 1976). Samples were kept at − 4 °C until analysis.
Immunofluorescence microscopy
Ten microliters of transformed E. coli culture was spotted onto an 8-mm slide well. The slide was air-dried and was fixed with methanol for 5 mins, post-washing with PBS, then 20 μL of fluorescein-conjugated monoclonal antibodies to the species-specific major outer membrane protein (PathfinderTMChlamydia DFA, Bio-Rad Laboratories, Inc., Redmond, WA, USA) was added. Later, the slide was incubated for 20 min at room temperature and was also washed with 5 mL PBS. Slides were screened at a magnification of ×400 and confirmed at ×1000 with a Carl-Zeiss epifluorescence AxioScope A1microscope.
SDS-PAGE and western blot
Recombinant Chlamydia MOMP (rMOMP) was purified and treated with 2× loading buffer, boiled for 5 min, and centrifuged for 5 min at 10,000g. Samples were later charged on to a 12% SDS-PAGE gel and were stained with Coomassie blue R stain (Sigma-Aldrich). For western blot, the proteins were electrophoretically transferred to 0.2 μm polyvinylidene difluoride (PVDF) membranes (Invitrogen Corp. Carlsbad, CA, USA) at 200 mA for 2.5 h. Membranes were blocked at room temperature for 2 h in PBS containing 3% bovine serum albumin (BSA), 0.05% Tween 20, and 0.02% sodium azide (NaN3; T-A-PBS). Then, 0.5 mL of positive control serum (not diluted) of C. trachomatis IgG IFA kit (Hemagen Diagnostics, Inc. Virgo® Products Division. Columbia, MD, USA) or 1:16 diluted serum specimens at 4 °C overnight was added. Blots were washed with T-A-PBS and incubated at room temperature for 2 h with goat anti-human IgG horseradish peroxidase–conjugated secondary antibody (Chemicon Int., Temecula, CA, USA). The blots were washed with PBS-Tween buffer (PBS-T), were rinsed with PBS, and were later developed with 4-chloro-1-naphthol and hydrogen peroxide (H2O2).
Serum study
In this study, serum of 40 infertile Mexican women was used. Sixteen were considered positive for C. trachomatis infection and 24 were negative for C. trachomatis infection, which were confirmed by the Chlamydia IgG antibody test (C. trachomatis/C. trach IgG IFA kit Hemagen Diagnostics, Inc.). The positive serum was of patients with infertility by tubal occlusion with titles of IgG antibodies against C. trachomatis higher than 1:64, and the negative serum was of patients without tubal occlusion with titles of IgG antibodies against C. trachomatis lower than 1:64. Written consent was obtained from all serum donors. Blood donor serum was collected at the central laboratory of National Institute of Perinatology of Mexico City.
Detection of serum antibody anti-C. trachomatis by ELISA
Human serum of infertility patients was analyzed by ELISA. Nunc™ C96 Maxisorp Immunoplates (Thermo Scientific Inc. Rockford, IL, USA) was coated with 100 μL of 1 μg/mL recombinant Chlamydia MOMP in 0.05 M carbonate-bicarbonate buffer (pH = 9.6) and refrigerated overnight. The plates were washed three times with 0.05% Tween-20 (Sigma-Aldrich) in PBS-T. The wells were blocked with 1% BSA (Sigma-Aldrich) in PBS-T at 200 μL/well for 1 h at room temperature and washed three times with PBS-T. In each well, 100 μL diluted human serum (1:128 in PBS-T) was added and incubated for 2 h at 37 °C. The plates were washed three times with PBS-T. Horseradish peroxidase–conjugated secondary antibodies (goat anti-human Ig, Chemicon) were diluted in PBS-T at 1:15,000; 100 μL of this diluted secondary antibody was added to each well and was incubated for 1 h at 37 °C. After incubation, the product was washed three times with PBS-T; subsequently, 100 μL of substrate solution (4 mg of orthophenylenediamine (OPD), 8 μL of H2O2 (30%), and 10 mL of citrate buffer) was added to each well, and the plates were incubated for 30 min at room temperature. Following incubation, the enzymatic reaction was stopped by adding 50 μL of 2.5 M sulfuric acid solution to each well. The optical density (OD) was determined using a 490-nm filter (Elx808, Biotek, Winooski, VT, USA).
For the analysis of the results, Eq. (1) was used.
The signal-to-cutoff (S/Co) is calculated by subtracting the absorbance value of the samples with the absorbance value of the negative control between the difference of subtracting the absorbance value of the positive control and the absorbance value of the negative control. Samples with absorbance values greater than or equal to the cutoff were considered positive.
Chlamydia IgG antibody test
The Chlamydia IgG antibody test (CAT) is an indirect fluorescent antibody (IFA) technique that enables observation of the antigen-antibody binding, detected secondarily by fluorescein-conjugated anti-globulin, to the corresponding antibody molecules. A 1:2 serial dilution of the serum specimens was made in PBS (pH 7.2); the 1:128 diluted specimens were placed on slides of the C. trachomatis/C. trach IgG IFA kit (Hemagen Diagnostics, Inc.). The development of CAT was carried out following manufacturers’ instructions. Slides were read on a Carl-Zeiss epifluorescence microscope with a ×100 objective. The endpoint titer was the serum dilution that gave a definite uniform bright-green fluorescence of Chlamydia particles within inclusions in the cytoplasm of infected cells.
Computer programs for molecular biology
Resulting nucleotide and amino acid sequences were aligned and analyzed with BioEdit v.7.0.4 program (Tom Hall, Ibis Therapeutics, Carlsbad, CA, USA). Homologous sequences were identified by the basic local alignment search tool (BLAST) from the genetic and amino acid sequence as templates in the database of GenBank (National Center for Biotechnology Information advances science and health (NCBI-NIH)).
Statistical method
The sensitivity and specificity of the ELISA test were evaluated by means of the 2 × 2-table method.
Results
Using the primer sets P3 and P4, an 879-bp DNA fragment, partially including the ompA gene, was amplified and cloned into the pGEM-T vector. The nucleotide sequence of the gene was analyzed and compared to the GenBank database for serovar L2 of C. trachomatis. The results obtained had 98% similarity (808/809), indicating that ompA gene was correctly amplified from serovar L2 of C. trachomatis.
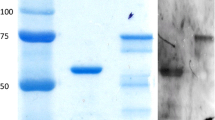
For the expression of the ompA gene, the PCR product was inserted into the PGEX-6P vector and E. coli BL21 was transformed obtaining the ompLGV construction. Several clones were reviewed and the one that produced the highest concentration of protein was chosen. A 12% SDS-PAGE assay showed that the ompA gene was well transcribed in the recombinant strain (protein of 29 kDa, approximately), but was not detected in the transcripts of a control transformant E. coli BL21 (PGEX-6P; Fig. 1).
Molecular cloning and expression of Chlamydia trachomatis major outer membrane protein in Escherichia coli. a Cloning an 879-bp fragment from the ompA gene. Lane 1: Amplicon of 879 bp. Lane 2: DNA molecular size marker. b Purification of recombinant protein by means of affinity chromatography. Lane 1: Total extract of E. coli BL21 transformed with ompLGV construction; Lanes 2 and 3: First wash followed by a second wash; Lanes 4–6: Eluted protein, and Lane WM is a molecular weight marker. cE. coli BL21 stained with a monoclonal antibody against major outer membrane protein from C. trachomatis conjugated with isothiocyanate of fluorescein. dE. coli BL21 not stained with a monoclonal antibody (negative control)
The result indicates that the cloned ompLGV construction was adequately expressed in E. coli BL21. Furthermore, a fluorescein-conjugated monoclonal antibody against the MOMP from C. trachomatis evidenced the presence of this protein in the recombinant BL21 strain (Fig. 1).
Establishment of indirect ELISA
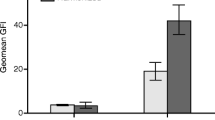
The optimal conditions of indirect ELISA were the following: antigen concentration at 1 μg/well and serum dilution, 1:128 (Table 1), with an optimal S/Co value of 1.4. Forty serum samples were tested under these conditions, and the S/Co value was calculated as low as 1.2 (Fig. 2). All tests were performed with three replicates. Twenty-six serum samples with S/Co ≥ 1.4 values were considered as serum with anti-Chlamydia antibodies, whereas serum samples with S/Co < 1.4 values were considered as serum without anti-Chlamydia antibodies (Fig. 2; Table 1). ELISA test results were compared with CAT test results. Of 40 specimens, 16 were true-positive, whereas 14 were true-negative and 10 were negative false by CAT test according to ELISA test results. The sensitivity and specificity of the ELISA with respect to the CAT test were 100 and 58.3%, respectively (Table 1).
Immunodetection of recombinant major outer membrane protein of Chlamydia trachomatis. a Western blot analysis. Lane 1: Positive control serum (Chlamydia trachomatis/C. trach IgG IFA kit from Hemagen Diagnostics, Inc); Lanes 2–5: Serum of patients with C. trachomatis infection; and Lanes 6–8: Serum of patients without C. trachomatis infection. b Response of antibodies against rMOMP from C. trachomatis of infertile women. Response of total antibodies evaluated by ELISA test with 1:128 diluted serum from infertile women. Obtained signal-to-cutoff (S/Co) ratios were the measurement of the signal strength of the sample and the signal strength of an internal cutoff
Detection of antibodies against recombinant MOMP by western blot
Twenty-eight serum samples analyzed by ELISA test were evaluated by western blot. Thirteen of them were positive and 15 were negative to western blot testing. Sera reacted predominantly with antigens ranging between 29 and 32 kDa. The Chlamydia-positive control serum from the CAT test showed antibodies against 29, 32, and 83 kDa antigens, whereas 5/10 positive sera to ELISA and CAT tests showed antibodies against the 29 kDa antigen and the other five sera showed antibodies against the 32 kDa antigen. Three negative false sera to CAT test showed antibodies against 29 kDa protein, while the negative sera to ELISA and CAT tests did not show antibodies against 29 or 32 kDa antigens (Fig. 2).
Discussion
The micro-immunofluorescence test (MIF) has been considered the gold standard in the serological diagnosis of Chlamydia infection. This test has several disadvantages that include the requirement of the elementary bodies of each of the serotypes of C. trachomatis, laborious technique, observer-dependent reading, and significant interlaboratory variation (Baud et al. 2010; Land et al. 2003). On the other hand, the ELISA test has been claimed to be highly sensitive and specific, because of these several assays have become commercially available in which specific synthetic peptides or recombinant proteins directed to MOMP are used (Bandehpour et al. 2006; Baud et al. 2010; Land et al. 2003; Mygind et al. 2000). In spite of the above, in many of the cases, these tests have not showed great sensitivity and specificity to identify infertile women with active infection by Chlamydia (Baud et al. 2010; Land et al. 2003). Due to the above, an ELISA test of greater sensitivity and specificity is required.
Recombinant protein production in E. coli system is a common practice in many laboratories (Schumann and Ferreira 2004; Khan et al. 2016). MOMP from C. trachomatis is a highly immunogenic antigen with a full-length of 39 kDa and is the major predominant protein on the surface (Findlay et al. 2005; De Haro-Cruz et al. 2011). In this study, a construction named ompLGV was performed with the variable segments VS1, VS2, VS3, andVS4; thereby, we ensured that an effective and specific response was achieved. The DNA sequencing of ompLGV construct confirmed the presence of the ompA gene, and sequencing showed 98% identity with the nucleotide sequence and 97% with the amino acid sequence of C. trachomatis LGV 2 (GenBank accession number, KP120855.1). The molecular weight calculated was 28.9 kDa according to the amino acid sequences. Once purified by affinity chromatography, SDS-PAGE gel indicated the presence of proteins with weights of 17, 29, and 62 kDa, approximately. Because rMOMP is tagged with GST and GST is a protein of 26 kDa, the size of our recombinant protein was estimated to be of 55 kDa. However, for reasons that have not been fully characterized in the literature, the structure of the GST fusion tag often degrades upon denaturation and reduction for SDS-PAGE gel electrophoresis (this data was furnished by the supplier of GST-tagged proteins products, Thermo Fisher Scientific, Waltham, MA, USA). Due to the above, the 29 kDa protein was considered as the rMOMP of C. trachomatis as it was very similar to the molecular weight estimated in silico for the obtained amino acid sequences (28,924 Da, according to the Expasy peptide mass software) of the ompA gene fragment of 879 bp. The 62 kDa protein may correspond to a complex of proteins between GST and rMOMP or the formation of a complex of rMOMP since this protein has many cysteine amino acids (Sardiu et al. 2007). Wen et al. (2016) observed MOMP dimer formation during recombinant MOMP expression in E. coli strains, and Sun et al. (2007) described the high stability of the trimeric form of the MOMP protein of C. trachomatis to SDS at temperatures ranging from 4 to 37 °C and over a pH range of 5–8; also the trimers of MOMP showed resistant to digestion with trypsin.
On the other hand, the fluorescein-conjugated monoclonal antibody against C. trachomatis confirmed the recombinant MOMP-expressing E. coli when induced with IPTG.
To confirm that the 29 kDa protein is the rMOMP of C. trachomatis, a western blot analysis was carried out. The Chlamydia-positive control serum of the CAT test identified the 29, 32, and 83 kDa bands that suggested these proteins as rMOMP of C. trachomatis. The 29 kDa protein has been considered as the rMOMP, because it is the protein with the greatest concentration obtained during purification. However, the 32 kDa protein may represent the rMOMP obtained from the finished MOMP synthesis. The 83 kDa band could be the trimeric form of MOMP linked with the GST-tagged because it was identified by the positive control serum. However, this data needed to be confirmed.
Afterward, rMOMP was used to develop an ELISA test to be used as a serological test to identify women with anti-C. trachomatis IgG antibodies. Several ELISA assays have been commercially developed with Chlamydia antigens; some of them are known to be C. trachomatis–specific. Menon et al. (2016) reported that CT-IgG ELISA-plus MEDAC assay (uses a synthetic peptide of a variable domain from an immunodominant region of the MOMP), as a serological test of C. trachomatis, significantly associated sub-fertility, with 50% of infertile women being positive. Land et al. (2003) observed a sensitivity and specificity of 55 and 83%, respectively, for the MEDAC test, while the ELISA Labsystems assay showed a sensitivity and specificity of 37 and 87%, respectively. The ELISA Labsystems assay uses synthetic peptides from MOMP of C. trachomatis. Mygind et al. (2000) used recombinant MOMP polyantigens in their ELISA test and showed a sensitivity of 80% and a specificity of 90%. These publications suggest that the accurate ELISA test is one that uses the recombinant MOMP from C. trachomatis.
The ELISA test of this study showed an adequate sensitivity (100%) and specificity (58.3%) that can be used in the detection of anti-Chlamydia antibodies compared to the CAT test. However, in comparison with Mygind’s ELISA test, the sensitivity of the ELISA test used in this experiment was better, while the specificity was lower (Mygind et al. 2000). The low specificity obtained might be linked to the CAT test that only identified the IgG against Chlamydia, whereas the ELISA test was seen to identify the total immunoglobulins against this bacterium. Another possibility is the larger sensitivity shown by ELISA test in comparison to the immunofluorescence assays since it used the rMOMP protein with its four variable regions. On the other hand, the low specificity was linked to the three different conformational structures of the rMOMP protein (83, 32, and 29 kDa), wherein only one could be recognized by antibodies against C. pneumoniae or C. psittaci. The purification of each protein and its analysis with an ELISA test will help in the identification of the protein that will aid to increase the specificity.
Furthermore, it is necessary to consider the cross-reactivity of the IgG antibodies against C. pneumoniae or C. psittaci could be shown by the sera collected and which can be detected by immunofluorescence or ELISA tests. Hernández-Trejo et al. (2014) have made evident that 46% of Mexican pregnant women have antibodies against C. trachomatis, C. psittaci, and C. pneumoniae; hence, this could also be a reason for the hindrance of the diagnoses of C. trachomatis infection. On the other hand, Baud et al. (2010) identified that three tests of ELISA for C. trachomatis diagnose showed cross-reaction with C. psittaci (p < 0.001) and one of them with C. pneumoniae. Similarly, Jones et al. (2003) showed poor specificity (5.6%; 95% CI, 0.68–18.7%) in C. trachomatis wherein an ELISA test (Genzyme Virotech) was reactive with 34 of 36 C. psittaci/C. pneumoniae–positive sera.
C. psittaci is a bird pathogen that causes a zoonosis mainly in people with occupational or recreational exposure, while C. pneumoniae is a human pathogen that causes infections of the respiratory tract, such as pneumonia and asthmatic bronchitis. The serological prevalence is not more than 14 and 86%, respectively (Fenga et al. 2007; Cui et al. 2018). Both Chlamydia species show cross-reactivity with antigens such as lipopolysaccharide (LPS), MOMP, outer membrane protein 2 (Omp2), or heat shock protein 60 (Hsp60) of C. trachomatis (Rahman et al. 2018a). In patients attending genitourinary clinics, it has been described that the cross-reactivity between C. trachomatis and C. pneumoniae is about 25.1%, while with C. psittaci, it is 0.1% (Moss et al. 1993).
On the other hand, a false negative is a test result that indicates a person does not have a disease or condition when the person actually does have it. The western blot analysis showed that true-positive sera identified 29 and 32 kDa proteins, while the three negative false sera to the CAT test showed antibodies against 29 kDa protein and only one of them showed antibodies against 32 kDa protein. With these results, we considered that maybe the ELISA test had a better specificity than CAT test and that could possibly not have a 58% specificity (Land et al. 2003). Due to the above, it is necessary to carry out a study with sera from people infected with C. pneumoniae and C. psittaci to evaluate the actual specificity of the ELISA test with the recombinant MOMP.
Strengths and limitations
The strength of this ELISA test was that it recorded a specificity of 100%, which was seen to enhance the detection of patients with C. trachomatis infection compared to the MOMP-MEDAC, Labsystem, and Savyon tests that show sensitivities between 55, 37, and 51%, respectively, in patients with tubal pathology (Land et al. 2003), while in women with positive infection, the sensitivity is 53, 60, and 42% when the tests are used, respectively (Rahman et al. 2018b).
The weakness of the test was its low specificity in comparison with the MOMP-MEDAC, Labsystem, and Savyon tests that describe 87, 87, and 83% in patients with tubal pathology (Land et al. 2003), while women with a positive infection are to show 97, 94, and 91% (Jones et al. 2003), respectively. This test requires further research due to the low specificity shown by it. One aspect that was not tested in this study was the dilution of the serum. Increasing the dilution of the serum could possibly improve the specificity of the test; however, it could also alter the sensitivity of the test. Another aspect that remains to be studied is the use of human sera positive for infection by C. pneumoniae and C. psittaci for the purpose of knowing how much cross-reactivity exists with this recombinant protein. It would also be necessary to study different populations of patients with the different pathologies caused by C. trachomatis infection such as infertility, pelvic inflammatory disease, ectopic pregnancies, non-gonococcal urethritis, a sexually transmitted disease lymphogranuloma venereum, and in the sexually active population. In addition, the western blot test could be evaluated to see if it is better than the ELISA test.
Based on the above, this ELISA test could be useful for commercialization if one can detect C. trachomatis infection and differentiate it from infection by other Chlamydia species, as well as preserve the ability to detect antibodies produced at different stages of the life cycle and the different C. trachomatis serovars. Finally, the importance of serological tests to C. trachomatis infection diagnosis is the potential it has as an epidemiological tool to evaluate interventions, including screening and treatment, also might be used as a potential biomarker of disease (infertility due to occlusion of fallopian tube), as well as assess humoral immune response to new vaccine candidates.
Conclusion
In conclusion, the rMOMP with the four variable regions of C. trachomatis expressed in E. coli is useful in the detection of antibodies to Chlamydia by means of an ELISA test.
References
Bandehpour M, Seyed N, Shadnoush M, Pakzad P, Kazemi B (2006) Using recombinant Chlamydia major outer membrane protein (MOMP) in ELISA diagnostic Kit. Iran J Biotechnol 4:239–244. RetrievedMarch 26, 2019, http://www.ijbiotech.com/article_6978.html
Bastidas RJ, Elwell CA, Engel JN, Valdivia RH (2013) Chlamydial intracellular survival strategies. Cold Spring Harb Perspect Med 3:a010256. https://doi.org/10.1101/cshperspect.a010256
Batteiger BE, Lin PM, Jones RB, Van Der Pol BJ (1996) Species-, serogroup-, and serovar-specific epitopes are juxtaposed in variable sequence region 4 of the major outer membrane proteins of some Chlamydia trachomatis serovars. Infect Immun 64:2839–2841
Baud D, Regan L, Greub G (2010) Comparison of five commercial serological tests for the detection of anti-Chlamydia trachomatis antibodies. Eur J Clin Microbiol Infect Dis 29:669–675. https://doi.org/10.1007/s10096-010-0912-4
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cui J, Yan W, Xie H, Xu S, Wang Q, Zhang W, Ni A (2018) A retrospective seroepidemiologic survey of Chlamydia pneumoniae infection in patients in Beijing between 2008 and 2017. PLoS ONE 13:e0206995. https://doi.org/10.1371/journal.pone.0206995
De Haro-Cruz M, DeLeón-Rodriguez I, Escobedo-Guerra MR, López-Hurtado M, Arteaga-Troncoso G, Ortiz-Ibarra FJ, Guerra-Infante FM (2011) Genotyping of Chlamydia trachomatis from endocervical specimens of infertile Mexican women. Enferm Infecc Microbiol Clin 29:102–108. https://doi.org/10.1016/j.eimc.2010.08.014
Fenga C, Cacciola A, Di Nola C, Calimeri S, Lo Giudice D, Pugliese M, Niutta PP, Martino LB (2007) Serologic investigation of the prevalence of Chlamydophila psittaci in occupationally-exposed subjects in eastern Sicily. Ann Agric Environ Med 14:93–96
Findlay HE, McClafferty H, Ashley RH (2005) Surface expression, single-channel analysis and membrane topology of recombinant Chlamydia trachomatis major outer membrane protein. BMC Microbiol 26:5. https://doi.org/10.1186/1471-2180-5-5
Hernández-Trejo M, Herrera-González N, Guerra-Infante FM (2014) Serological evidence of infection by three species of Chlamydia in pregnant women. Ginecol Obstet Mex 82:585–590
Ishizaki M, Allen JE, Beatty PR, Stephens RS (1992) Immune specificity of murine T-cell lines to the major outer membrane protein of Chlamydia trachomatis. Infect Immun 60:3714–3718
Jiang P, Cai Y, Chen J, Ye X, Mao S, Zhu S, Xue X, Chen S, Zhang L (2017) Evaluation of tandem Chlamydia trachomatis MOMP multi-epitopes vaccine in BALB/c mice model. Vaccine 35:3096–3103. https://doi.org/10.1016/j.vaccine.2017.04.031
Jones CS, Maple PA, Andrews NJ, Paul ID, Caul EO (2003) Measurement of IgG antibodies to Chlamydia trachomatis by commercial enzyme immunoassays and immunofluorescence in sera from pregnant women and patients with infertility, pelvic inflammatory disease, ectopic pregnancy, and laboratory diagnosed Chlamydia psittaci/Chlamydia pneumoniae infection. J Clin Pathol 56:225–229
Khan S, Ullah MW, Siddique R, Nabi G, Manan S, Yousaf M, Hou H (2016) Role of recombinant DNA technology to improve Life. Int J Genomics 2016:2405954. https://doi.org/10.1155/2016/2405954
Land JA, Gijsen AP, Kessels AG, Slobbe ME, Bruggeman CA (2003) Performance of five serological chlamydia antibody tests in subfertile women. Hum Reprod 18:2621–2627
Menon S, Stansfield SH, Walsh M, Hope E, Isaia L, Righarts AA, Niupulusu T, Temese SV, Iosefa-Siitia L, Auvaa L, Tapelu SA, Motu MF, Suaalii-Sauni T, Timms P, Hill PC, Huston WM (2016) Sero-epidemiological assessment of Chlamydia trachomatis infection and sub-fertility in Samoan women. BMC Infect Dis 16:175. https://doi.org/10.1186/s12879-016-1508-0
Moss TR, Darougar S, Woodland RM, Nathan M, Dines RJ, Cathrine V (1993) Antibodies to Chlamydia species in patients attending a genitourinary clinic and the impact of antibodies to C. pneumoniae and C. psittaci on the sensitivity and the specificity of C. trachomatis serology tests. Sex Transm Dis 20:61–65
Mygind P, Christiansen G, Persson K, Birkelund S (2000) Detection of Chlamydia trachomatis-specific antibodies in human sera by recombinant major outer-membrane protein polyantigens. J Med Microbiol 49:457–465
O’Connell CM, Ferone ME (2016) Chlamydia trachomatis genital infections. Microb Cell 3:390–403. https://doi.org/10.15698/mic2016.09.525
Rahman KS, Darville T, Russell AN, O’Connell CM, Wiesenfeld HC, Hillier SL, Chowdhury EU, Juan YC, Kaltenboeck B (2018a) Discovery of human-specific immunodominant Chlamydia trachomatis B cell epitopes. mSphere. 3:e00246–e00218. https://doi.org/10.1128/mSphere.00246-18.
Rahman KS, Darville T, Russell AN, O’Connell CM, Wiesenfeld HC, Hillier SL, Lee DE, Kaltenboeck B (2018b) Comprehensive molecular serology of human Chlamydia trachomatis infections by peptide enzyme-linked immunosorbent assays. mSphere 3:e00253–e00218. https://doi.org/10.1128/mSphere.00253-18.
Sardiu ME, Cheung MS, Yu YK (2007) Cysteine-cysteine contact preference leads to target-focusing in protein folding. Biophys J 93:938–951
Schumann W, Ferreira LCS (2004) Production of recombinant proteins in Escherichia coli. Genet Mol Biol 27:442–453
Sun G, Pal S, Sarcon AK, Kim S, Sugawara E, Nikaido H, Cocco MJ, Peterson EM, de la Maza LM (2007) Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J Bacteriol 189:6222–6235
Wen Z, Boddicker MA, Kaufhold RM, Khandelwal P, Durr E, Qiu P, Lucas BJ, Nahas DD, Cook JC, Touch S, Skinner JM, Espeseth AS, Przysiecki CT, Zhang L (2016) Recombinant expression of Chlamydia trachomatis major outer membrane protein in E. coli outer membrane as a substrate for vaccine research. BMC Microbiol 16:165. https://doi.org/10.1186/s12866-016-0787-3
Yang CL, Maclean I, Burnham RC (1993) DNA sequence polymorphism of the Chlamydia trachomatis omp1 gene. J Infect Dis 168:1225–1230
Author information
Authors and Affiliations
Contributions
The authors have seen and approved the manuscript being submitted.
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Furthermore, Informed consent was obtained from all individual participants involved in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Haro-Cruz, M.J., Guadarrama-Macedo, S.I., López-Hurtado, M. et al. Obtaining an ELISA test based on a recombinant protein of Chlamydia trachomatis. Int Microbiol 22, 471–478 (2019). https://doi.org/10.1007/s10123-019-00074-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-019-00074-4