Abstract
The tumor suppressor gene phosphatase and tensin homolog (PTEN) in PI3K/Akt/mTOR pathway is essential in inhibiting tumor growth and metastasis. However, whether the mutation of PTEN gene could induce tumorigenesis and impact the treatment of gastric cancer is still unclear. The purpose of the study was to investigate the combined treatment of gastric tumorigenesis using Rapamycin and Fluorouracil (5-Fu) through interfering with the Akt/mTOR pathway in a mouse model with PTEN conditional deletion. Three groups of mice were exposed for 5 days to Rapamycin and 5-Fu separately and together. The gene expression of the Akt/mTOR pathway, the protein expression of caspase-3 and p-Akt, p-S6K and p-4EBP1, and the pathological changes in stomachs were analyzed. Our study demonstrates that the conditional PTEN deletion in the cells of glandular stomach induces hyperplastic gastric tumors in mice. The combined Rapamycin administration with 5-Fu resulted in better outcomes than their separate administration for the treatment of gastric cancer by inhibiting the mTOR signal pathway. Our study indicates that Rapamycin has a synergistic interaction with chemotherapeutic 5-Fu, and demonstrates a potential therapeutic combination treatment on glandular stomach tumor with PTEN functional absence or aberrantly activated Akt/mTOR pathway. It provides important insights into the inhibition of the Akt/mTOR pathway in gastric cancer clinical therapy.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is the fifth most common malignancy and remains the third cause of death worldwide [1]. Established clinical treatments and interventions (e.g., surgical resection) are not fully successful in treating GC [2]. Although adjuvant therapeutic strategies, such as chemotherapy and radiotherapy greatly improve the prognosis for GC patients, the 5-year overall survival rate is still relatively low (< 30% worldwide) [3, 4]. There is an urgent need to find new therapeutic strategies to cure GC.
Tumorigenesis involves a series of genetic events that disrupt or alter signaling networks controlling cell proliferation and survival. Multiple cell signaling pathways, including G-protein-coupled receptors signaling to MAPK/Erk, p53pathway, NF-κB, and caspase-3 pathway, as well as phosphoinositide 3-kinase (PI3K)/Akt/ Mammalian Target of Rapamycin (mTOR) pathway, are involved in the tumorigenesis [5,6,7,8,9,10,11,12,13,14,15,16,17]. The PI3K/Akt pathway can be considered as a central integrator of a tangled web of signaling networks with direct and indirect effects on each other [18]. PI3K/Akt activation can produce diverse changes in cell physiology and inhibits apoptosis, indicating that the PI3K/Akt pathway mutation may have broad consequences on tumorigenesis and treatment responses [17, 18].
The mTOR protein is a 289-kDa serine-threonine kinase that belongs to the PI3K-related kinase family and is conserved throughout evolution. Studies show that the mTOR signaling pathway is activated during various cellular processes (e.g., tumor formation and angiogenesis, insulin resistance, adipogenesis, and T-lymphocyte activation) and is deregulated in humans who have diseases such as cancer and type 2 diabetes [7, 19, 20]. Though PI3K/Akt/mTOR pathway plays an important role in the development of human gastric tumorigenesis [21], whether a mutation in a specific component of the PI3K/Akt pathway as a single factor could produce the same effects on tumor physiology or induce tumorigenesis is still unclear.
The PI3K/Akt pathway has a natural inhibitor called Phosphatase and tensin homolog deleted on chromosome ten (PTEN) whose function is to limit proliferation in cells, helping to prevent cancer [22]. One well-known impact of PTEN loss on cell metabolism is increased protein synthesis through modulation of two important components of the protein synthesis machinery, eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) and p70S6 kinase, two downstream effects of raptor-mTOR (mTORC1) [23]. PTEN works by dephosphorylating PIP3 to PIP2 which limits Akt’s ability to bind to the membrane, decreasing its activity. PTEN gene can be found mutated frequently both very early in tumorigenesis (as in hereditary cancer syndromes) and also much later in advanced cancers [24]. Previous studies have demonstrated that the specific inactivation of the PTEN alleles in mouse gastric epithelium induced an aberrant Akt-p53-miR365-cyclin D1/cdc25A pathway, which ultimately initiated spontaneous gastric tumor [25,26,27,28]. Considering the important role of the Akt/mTOR pathway [29,30,31], it is necessary to investigate the status of this pathway in the glandular stomach during the process of tumorigenesis initiated by PTEN alleles conditional deletion in mice gastric epithelia.
Moreover, fluorouracil (5-Fu) is widely used as an important first-line anti-cancer drug in clinical use, while the drug resistance of 5-Fu to patients with gastric cancer has become a serious therapeutic problem [32]. The mTOR pathway has long been identified as the target of the antibiotic Rapamycin [33]. Previous studies have shown that the PI3K/Akt/mTOR pathway plays an important role in mediating drug resistance in many kinds of human cancers [34,35,36,37]. We hypothesized that specific inhibition of the Akt/mTOR pathway or combination with 5-Fu could become an effective therapeutic strategy for gastric cancer therapy.
To test our hypothesis, we first developed a novel mouse model with PTEN conditional deletion to find whether PTEN gene deletion can induce Akt/mTOR pathway activation directly leading to gastric tumor genesis. We then use the immune impresser, Rapamycin combined with 5-Fu to improve the tumor treatment with the advantages of chemo-sensitivity, lower metastasis, and lower proliferation (Fig. 1). Our study provides important insights into the inhibition of the Akt/mTOR pathway in gastric cancer clinical therapy.
Results
Genotyping for the hyperplastic gastric tumors induced by PTEN conditional deletion
The Cre+;PTENfl/fl mice with glandular stomach cells’ specific PTEN alleles deletion developed hyperplastic glandular stomach tumor in an early age (before postnatal 45 days) (Fig.S1). Tumorigenesis in glandular stomach resulted in a progressive decrease in the survival of the SP-A-Cre+;PTENfl/fl mice, with a median survival time of (58 ± 1.9) days (n = 15) (Fig. S1). The tumor is mainly characterized by irregular proliferation of the glandular stomach. Gender did not introduce any differences in the prevalence of tumorigenesis (data not shown). No obvious differences in phenotypes among mice with different gene types upon birth, while the bodyweight of the Cre+;PTENfl/fl mice at the 45th postnatal day was lower than the Cre—;PTENfl/fl mice with the same gender and same age (Fig. S1).
Akt/mTOR pathway activation in the hyperplastic gastric tumors induced by PTEN conditional deletion
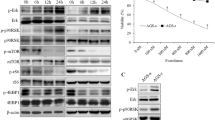
RT-PCR results for the glandular stomach exhibited the activation of the Akt/mTOR pathway at gene level as indicated in Fig. 2B and Fig. S2. The direct activators of mTOR and its upstream regulator gene, Akt were found to be significantly increased in the Cre+;PTENfl/fl mice (p < 0.05) as compared to the littermate control Cre−;PTENfl/fl, although 4E-binding protein (4EBP1) and p70S6 kinase (S6K), another two downstream effectors, did not display a significant change (Fig. 2B).
Gastric epithelial specific PTEN alleles deletion induced aberrant constitutional activation of Akt/mTOR pathway in glandular stomach. A, B The expression of Akt/mTOR pathway relative genes, including Akt, Rheb, mTOR, S6K, and 4EBP1 in glandular stomach of Cre+;PTEN fl/fl mice and littermates control Cre−;PTEN fl/fl mice, was detected by semi-quantitative RT-PCR, densitometry was quantified using the Image J software. C, D The protein levels of p-Akt (Ser473), p-S6K (Thr389), and p-4EBP1 (Thr37/46) in glandular stomach of Cre+;PTENfl/fl mice and littermates control Cre−;PTENfl/fl mice were assayed by western blot. All tests were done when mice were at 45-day-old. The grouping of gene blots was cropped from different parts of the same gel (Fig. S2). The grouping of protein blots was cropped from different gels (Fig. S3). Data were shown as the mean ± SD of at least three replicates. *P < 0.05, **P < 0.01, versus wild type Cre−;PTENfl/fl mice
The phosphorylated level of p-Akt (p-Akt, Ser473), p-S6K (Thr389), and p-4EBP1 (Thr37/46) in the glandular stomach of the S-PA-Cre+;PTENfl/fl mice are shown in Fig. 2C, D (Fig. S3). At the protein level, p-Akt and p-4EBP1 showed a significant increase (p < 0.05) in the Cre+;PTENfl/fl mice compare to the Cre−;PTENfl/fl mice as indicated in Fig. 2C, D, while S6K showed no statistically significant increase (P > 0.05). The results indicate that the activation of the Akt/mTOR pathway happens at the protein level.
Reversal of PTEN conditional deletion induced glandular stomach tumorigenesis and the attenuation of aberrant Akt/mTOR pathway activation after Rapamycin administration
We hypothesized that the pharmacological inhibition of mTOR has anti-tumor effects, especially for tumors accompanied by PTEN functional mutation or Akt signaling aberrant activation. We found that administration of Rapamycin alone can rescue the hyperplastic and metaplastic changes in Akt/mTOR pathway (Fig. 3), with a moderately prolonged survival time (the median survival time of mouse with gastric cancer increased significantly as compared to the control group [(72 ± 5.3) days versus (59 ± 3) days)] (Fig. 3B). Histological analysis showed that Rapamycin treatment inhibited the proliferation of the glandular stomach tumor cells, promoted normal differentiation, as well as induced increased cell apoptosis in epithelium mucosae (morphologically distinct cells were counted as apoptotic cells) (Fig. 3A). Moreover, a significant reduction of the inflammatory cell infiltration in the gastric glandular stomachs of the Rapamycin-treated SP-A-Cre+;PTENfl/fl mice (Fig. 3A—-b versus A—c) was observed. Our results show that intraperitoneal Rapamycin administration alone led to modest tumor regression and significantly amended the damaged stomachs of the mice with gastric epithelial specific PTEN alleles deletion when compared to the controls (Fig. 3A—-a/b versus A—c/d).
Rapamycin reduced the malignancy degree and inhibited proliferation of the gastric epithelial cells, attenuated the activation of Akt/mTOR pathway, and prolonged survival time of the SP-A-Cre+;PTENfl/fl mice with gastric glandular carcinoma induced by conditional PTEN absence. A The pathological changes of stomachs for control (c, d) and after 5 days’ Rapamycin treatment (a, b). The mice were dissected and the stomachs were collected for histology and immunohistochemistry analysis. Rapamycin alone treatment promoted normal differentiation and apoptosis of the epithelium, and lastly, led to modest tumor regression and significantly improved the damaged stomach (c versus a). d showed higher magnification of apoptotic cells in the epithelium mucosa. B The survival rate of mice was analyzed by the Kaplan–Meier survival analysis approach to generate a Kaplan–Meier survival plot using SPSS17.0. The median survival time of Rapamycin untreated and treated group was (59 ± 3) days (n = 16) and (72 ± 5.3) days (n = 15), respectively (P < 0.01). *P < 0.05, ** P < 0.01, versus the littermate control Cre−;PTENfl/fl mice. C, D Protein level of p-Akt, p-S6K, and p-4EBP1 of Rapamycin-treated group and the untreated group was detected by western blot. The grouping of protein blots was cropped from different gels (Fig. S5). *P < 0.05, Rapamycin treatment group versus untreated group
The immunohistochemistry staining with Ki67 and 4EBP1 showed that administration of Rapamycin alone significantly reduced proliferation of the gastric epithelial cells and attenuated invasion (Fig. S4—c/d versus a/b), which suggests that the effect of Rapamycin on gastric cancer can be directly or indirectly related to the inhibition of cell proliferation. To determine the inhibition of Rapamycin on mTOR, proteins expression related to the primary glandular stomach tumor including p-Akt, p-S6K, and p-4EBP1 were detected by western blot (Fig. 3C, D) (Fig. S5). As expected, Rapamycin administration rescued the aberrant overexpression of p-S6K and p-4EBP1 in the stomach of SP-A-Cre+;PTENfl/fl mice. Notably, the level of p-Akt was also reduced significantly, which indicated the down-regulation of its activity.
Reversal of the glandular stomach tumorigenesis and attenuation of aberrant activation of the Akt/mTOR pathway by combined Rapamycin and 5-Fu administration
Administration of 5-Fu alone did not change the malignant phenotype of the gastric cancer mice in the Akt/mTOR pathway (Fig. 4). The aberrant level of the p-Akt, p-S6K, and p-4EBP1 are also the same with and without administration of 5-Fu (Fig. 4C, D) (Fig. S6). This finding suggested that PTEN conditional deletion induced the abnormal activated Akt/mTOR pathway cannot be rescued by 5-Fu administration. Besides, the median survival time (61 ± 2.0) days by 5-Fu administration was the same as the control group with vehicle (60 ± 2.6) days (Fig. 4B). However, there was a significant increase in the activated caspase-3 (Fig. 3C, D), which likely suggests increased cell apoptosis. These results suggest the drug failure of 5-Fu may be responsible for the ineffective inhibition of the Akt/mTOR pathway activated by the PTEN deficiency.
The therapeutic effect of 5-Fu was attenuated in SPA-Cre+;PTENfl/fl mice with gastric glandular carcinoma induced by conditional PTEN absence. A 5-Fu alone administration did not change the phenotype of the glandular stomach tumor by histological analysis (c and d versus a and b). B The median survival time of 5-Fu untreated and treated group was (60 ± 2.6) days (n = 12) and (61 ± 2.0) days (n = 17), respectively (P > 0.05). The results showed no significant difference. C, D The protein level of p-Akt, p-S6K, p-4EBP1, and caspase-3 of the 5-Fu treated and the untreated group were detected by western blot. The results showed 5-Fu alone treatment did not change the aberrant Akt/mTOR pathway activation except for a significant increasing of caspase-3. The grouping of protein blots was cropped from different gels (Fig. S6). **P < 0.01, 5-Fu treatment group versus untreated group
The administration of Rapamycin combined with 5-Fu led to modest tumor regression and significantly slowed down the damages to stomachs in SP-A-Cre+;PTENfl/fl mice as compared to the control (Fig. 5). The combined administration attenuated the malignance characteristics (such as the poorly differentiated and over-proliferation phenotype and tumor invasion states) of the glandular stomach tumor significantly and almost restored the abnormal histopathological changes of gastric mucosa after 5 days of’ treatment (Fig. 5A). The levels of p-Akt and p-4EBP1 are significantly decreased after the combined administration (Fig. 5C, D) (Fig. S7). Our results suggest that combined administration effectively inhibited the aberrant activation of the Akt/mTOR pathway in the glandular stomach of SP-A-Cre+;PTENfl/fl mice. Besides, in comparison with the control group and with the groups where 5-Fu or Rapamycin were administered alone, the median survival of SPA-Cre+;PTENfl/fl mice treated with a combination of Rapamycin and 5-Fu increased significantly [(79 ± 5.3) days, Figs. 3B, 4B, 5B]. No significant increase of the activated caspase-3 was observed, which suggested that cell apoptosis was likely restored compared with 5-Fu alone administration (Fig. 5C, D).
Combined treatment with Rapamycin and 5-Fu showed significant improvement on the malignance of the glandular gastric cancer in SP-A-Cre+;PTENfl/fl mice. A Combined administration significantly alleviated the malignant phenotype of the glandular stomach tumor by histological analysis (c and d versus a and b). Scale bar in a and c was 500 μm, and in b and d was 200 μm. B The median survival time of the combined untreated group and treated group was (62 ± 0.7) days (n = 18) and (79 ± 5.3) days (n = 28) respectively (P < 0.01). That of the combined treated group was significantly longer. C, D Protein levels of p-Akt, p-S6K, p-4EBP1, and caspase-3 in the gastric tumor of combined treated and the untreated group were detected by western blot. The results showed combined administration significantly decreased the expression of p-Akt and p-4EBP1 in SP-A-Cre+;PTENfl/fl mice. The grouping of protein blots was cropped from different gels (Fig. S7). **P < 0.01, drug combination treatment group versus the vehicle control group
Discussion
Our study developed a novel gastric tumorigenesis model induced by conditional PTEN alleles deletion. The model shows that the proliferation of glandular stomach’s cells was increased, accompanying by poorly differentiated and impaired gastric epithelium mucosae. These results indicate that conditional deletion of PTEN alleles in the glandular stomach as a single genetic factor could lead to aberrant activation of the Akt/mTOR pathway in the glandular stomach of the SP-A-Cre+;PTENfl/fl mice. We also found macrophages in the process of the glandular stomach tumorigenesis, which suggests the relevance of the inflammation to gastric cancer development.
Protein phosphorylation is a reversible post-translational modification of proteins. Phosphorylation alters the structural conformation of a protein, causing it to become activated, deactivated, or modifying its function [38]. In the gastric tumorigenesis model, Akt was activated significantly at the gene and phosphorylated protein level, which provides additional evidence that an excessively activated Akt has broader consequences for tumorigenesis. This result is consistent with the clinical studies which have shown that regulation of Akt expression and phosphorylation in an appropriate range is important for maintaining normal cell growth, preventing tumorigenesis and tumor drug resistance to conventional chemotherapies [18, 39].
As a key downstream substrate of p-Akt, the mTOR activation stimulates protein synthesis and promotes cell proliferation in different organs and systems [19, 29, 30, 40]. Eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) is a member of a family of translation repressor proteins, and a well-known substrate of the PI3K/Akt signaling pathway. Phosphorylation of 4EBP1 causes its release from eIF4E to allow cap-dependent translation to proceed. Wendel and his colleagues described that eIF4E showed a clear potent oncogene and phenotypes consistent with an anti-apoptotic gene by effecting mRNA 5′cap [41].
P-S6K is an enzyme (specifically, a protein kinase) that in humans is encoded by the RPS6KB1 gene. It is a serine/threonine kinase that acts downstream of PIP3 and phosphoinositide-dependent kinase-1 in the PI3 kinase pathway. Studies have shown that even though p-S6K phosphorylation is important for glucose homeostasis and controlling cell size, it is not essential to the regulation of 5′ TOP mRNAs [29, 30]. Although both of p-S6K and p-4EBP play critical roles in translational regulation, in our study p-S6K displayed only a slight increase in the contrast to the significant increase of p-4EBP1 in the SP-A-Cre+;PTENfl/fl mice. This is consistent with our RT-PCR results [25]. The functional difference between the two genes’ expression may account for such a discrepancy between transcriptional and post-translational modification of the two downstream effectors of mTOR.
Our study shows that administration of Rapamycin alone moderately reduced the proliferation of epithelial cells and promoted transformation to the normal differentiation and gastric epithelium structure. The observed decrease in the levels of p-S6K and p-4EBP1 demonstrates the ability of Rapamycin to inhibit mTOR activity in vivo. Notably, administration of Rapamycin reduced the level of p-Akt significantly, which suggested the down-regulation of its activity. The inhibition of mTORC1 caused by Rapamycin likely lead to a reduction of the total TOR, and then impacts the amount or the activity of rictor-mTOR (TORC2), whereas the TORC2 complex directly phosphorylates Akt on the Ser 473 site in vivo and facilitates its phosphorylation of Thr 308 site by phosphoinositide-dependent kinase-1 [42, 43].
The administration of 5-Fu alone in the mice model with PTEN conditional deletion did neither present any improvement on the malignancy of the glandular stomach tumorigenesis nor prolong the survival time for the mice with gastric tumorigenesis with PTEN deficiency. Nevertheless, combined administration of Rapamycin and 5-Fu showed a significant therapeutic effect on the gastric tumorigenesis, effectively suppressed tumor growth and prolonged survival time, as well as showed a modest synergistic therapeutic effect on the mice with gastric tumorigenesis. This finding indicates that inhibition of the Akt/mTOR pathway could enhance the efficacy of chemotherapy and/or combat tumor resistance to 5-Fu in gastric tumorigenesis and thus constitutes an attractive target for cancer drug development [44].
Our results further indicate that tumor harboring activating mutations in the Akt/mTOR pathway or the loss of PTEN may be particularly sensitive to PI3K/Akt/mTOR pathway inhibitors. This finding provides new opportunities for developing personalized anti-tumor treatments. Also, previous studies found that human cancer results from multiple known oncogenic mutations. These concomitant oncogenic genetic changes might affect the tumors’ response to targeted inhibitors. Our finding demonstrates that the combined administration of Rapamycin and 5-Fu may become a useful treatment method to enhance the targeted inhibitors.
Clinical studies had shown that the loss or diminished expression of PTEN in malignant tissues of the stomach may be a major general event in the progression of GC. For example, Li et al. [45] reported that a reduction or loss of PTEN expression was detected in over half of GC cases (58.7%, 67/114). Zhu et al. [46], found that the percentage of PTEN expression in GC samples (48%, 77/159) was significantly lower compared with that in adjacent normal tissue (75%, 113/151). Yusufu et al. [47] also found that the PTEN expression in GC (34%) and metastatic lymph nodes (24.4%) was downregulated compared with in normal tissues (92.5%). Moreover, the infection of Helicobacter pylori (H pylori) may induce GC which was relevant with PTEN. For instance, CagA of H pylori can reduce the expression of PTEN by enhancing its promoter methylation [48, 49].
Concerning current clinical trials investigating mTOR inhibitors for cancer therapy, our study presented here highlights the possibility of Rapamycin being used in the PTEN mutation context for gastric cancer therapy. However, to promote the transformation to practical application, further studies are needed.
Methods
Mice generation and genotyping
Mice homozygous for PTEN conditional deletion on their glandular stomach was used as the gastric tumorigenesis model. Animal experiments were approved by the Animal Experiment Committee of the Institute of Disease Control and Prevention, Academy of Military Medical Sciences (China) by the guidelines of the Institutional Animal Care and Use Committee (IACUC2009ZX09501-2012). Mice were produced using the Cre-Loxp method. 129/SvJ;C57BL/6 mixed background mice was used for constructing the conditional PTEN deletion mouse [25]. Briefly, mice carrying conditional floxed PTEN (PTENfl/fl) alleles were bred with SP-A-Cre transgenic mice that express the Cre recombinase in gastric epithelium to produce Cre+;PTENfl/fl (PTEN conditional deletion) mice. The mice were housed in a 12 h light/dark cycle and temperature-controlled SPF environment. Mice identified with homozygous PTEN alleles absence (Cre+;PTENfl/fl) were used as the test system, while the heterozygous PTEN allele absence (Cre+; PTENfl/−) and those PTEN floxed mice (Cre—;PTENfl/fl) were used for the offspring breeding. The offspring were genotyped by PCR analysis.
Experimental setup and drug administration
The mice (45-day-old) were treated with Rapamycin, 5-Fu, and Rapamycin and 5-Fu combination for 5 days, respectively. Rapamycin (LC laboratories) was initially dissolved in 100% ethanol, stored at − 20 °C, and further diluted in an aqueous solution of 5.2% Tween-80 and 5.2% PEG-400 (final ethanol concentration 2%) immediately before use in the experiment. Five-Fu (Sigma) was dissolved in double distilled water to make the exposure concentrations of 2.5 mg/mL and 2.0 mg/mL, separately. For Rapamycin alone treatment (n = 15), Rapamycin (4 mg/kg) was administrated intraperitoneally for 5 days, while the vehicle was given as control (n = 16). For 5-Fu alone treatment, 5-Fu (25 mg/kg) was administrated intraperitoneally for 5 days (n = 17), while the placebo was given as control (n = 12). For the combination treatment, Rapamycin (4 mg/kg) was given on the first day, followed by the 5-Fu (20 mg/kg) on the second day intraperitoneally for 10 days (five times for each drug, respectively). The survival of mice was recorded every day.
RNA extraction and RT-PCR detection
Genomic DNA was isolated from tail tips of treated and control mice by standard procedures [25]. The genotyping was determined by PCR [25]. Total RNA was isolated from the glandular stomach of the Cre—;PTENfl/fl and Cre+;PTENfl/fl mice using Trizol reagent (Invitrogen, #15,596,026). The first-strand cDNA was synthesized from total RNA using the cDNA synthesis kit (Takara, #6110) according to the manufacturer's instructions, and RT-PCR was performed to evaluate the relative expression of target genes. The results were analyzed by Image J and were normalized to β-actin. The specific primers in Table 1 were used.
Histology and immunohistochemistry
To analyze the pathological changes of stomachs after 5 days’ drug treatment, the mice were dissected and the stomachs (treatment and control) were collected for histology and immunohistochemistry analysis. The collected stomachs were fixed in 10% neutral formalin for 24 h followed by a series of graded alcohol and xylene to dehydrate them, and embedded with paraffin for section. The stomachs were cut into 3 μm thick sections. For immunohistochemistry, formalin-fixed paraffin-embedded sections were de-paraffinized in xylene and rehydrated through graded alcohol to distilled water. The sections were subjected to antigen retrieval by boiling in a microwave for 5 min in 10 mM/L sodium citrate buffer (pH 6.0) and cooled on the benchtop for about 40 min, and then exposed to 3% hydrogen peroxide for 20 min at room temperature to block endogenous peroxidase activity. Then the sections were incubated in a blocking solution for 30 min at room temperature, incubated with Ki67 antibody (IMGENEX, #NB500-170) overnight at 4 °C, washed with PBS three times. After that, sections were incubated with biotinylated secondary antibody for 30 min and washed by PBS three times, and then sections were incubated with AB enzyme reagent according to the manufacturer’s instruction (Santa Cruz, #sc2019). After washing three times, the sections were treated with peroxidase substrate until the desired stain intensity was developed. Followed by 5 min washing with distilled water, sections were counter-stained with Gill’s formulation hematoxylin for 10 s and immediately washed with distilled water. Then they were dehydrated by alcohol and xylene, wiped to remove off excess xylenes, added 1–2 drops of permanent mounting medium, and cover with a glass coverslip.
Protein lyses and western blot
The stomach tissue was collected from the treated mouse and control. The collected tissues were lysed with modified RIPA buffer (Beyotime, R0010) and phosphatase inhibitor on ice for 5 min with gentle grinding and sonication for 8 s to shear DNA and reduce sample viscosity, then the lysates were centrifuged at 11,800 rcf for 20 min at 4 °C. The protein content of the supernatant was quantified using the BCA assay (Beyotime, #P0010S). Protein samples (60 μg) were loaded onto SDS–PAGE gel for electrophoresis, then the protein was transferred to PVDF membrane, which was blocked with 5% nonfat dry milk in TBST buffer (20 mM Tris–HCl (pH 7.4), 150 mM NaCl, and 0.1% Tween 20) and then incubated with primary antibody overnight at 4 °C. The membrane was washed three times with TBST buffer and incubated with secondary antibody for 1 h. Then the membrane was washed three times and detected using the enhanced chemiluminescence detection system (Santa Cruz, #sc2048). The phosphorylated level of Akt, S6K, and 4EBP1 and the protein level of p-Akt (Ser473), p-S6K (Thr389), and p-4EBP1 (Thr37/46) in the glandular stomach of the SPA-Cre+;PTENfl/fl mice were quantified by Image J 1.42 (NIH). Each experiment was repeated three times. The following antibodies were used for western blot: mouse monoclonal anti-β-actin, rabbit polyclonal anti-phosphorylated-p70S6K (Thr389) (sc-47778 and sc-11759, Santa Cruz), rabbit monoclonal anti-phosphorylated-Akt (Ser473) (4060, Cell Signaling Technology), rabbit monoclonal anti-phosphorylated-4EBP1 (Thr37/46) (2855, Cell Signaling Technology), and rabbit polyclonal anti-caspase-3 (9662, Cell Signaling Technology). The secondary antibodies are HRP AffiniPure Goat Anti-Rabbit IgG (H + L) (ZSGB-BIO, #ZB-2301) and HRP-labeled Goat Anti-Mouse IgG (H + L) (ZSGB-BIO, #ZB-2305). The dilution of the first antibodies used in immunohistochemistry and Western blot was 1:800 or 1:1000. The dilution of the second antibody is 1:2500. The diluted solution included 5% buffer solution of 1× TBS, TBS (Tris Buffered Saline, pH 7.2).
Statistical analysis
The survival rate of mice was analyzed by the Kaplan–Meier survival analysis approach to generate a Kaplan–Meier survival plot Using SPSS17.0. The data on gene expression of Akt/mTOR pathway, and protein expression of caspase-3 and p-Akt, p-S6K, and p-4EBP1, were analyzed using a two-tailed student’s t test or one-way ANOVA followed by Fisher’s least significant difference test by SPSS 17.0. Levene’s test was used to check the homogeneity of variances. The results were presented as means ± SD, and P < 0.05 was considered significant.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumor Biol. 2017;39(7):1010428317714626.
Yamashita K, Sakuramoto S, Nemoto M, Shibata T, Mieno H, Katada N, Watanabe M. Trend in gastric cancer: 35 years of surgical experience in Japan. World J Gastroenterol. 2011;17(29):3390.
Ye YW, Dong RZ, Zhou Y, Du CY, Wang CM, Fu H, Shi YQ. Prognostic analysis of familial gastric cancer in Chinese population. J Surg Oncol. 2011;104(1):76–82.
Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6(12):909–23.
Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene. 2007;26(22):3122–42.
Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9–22.
Löbrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer. 2007;7(11):861–9.
Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–74.
McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26(22):3113–21.
Zhang H, Bajraszewski N, Wu E, Wang H, Moseman AP, Dabora SL, Kwiatkowski DJ. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Investig. 2007;117(3):730–8.
Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y. The RAS/MAPK syndromes: novel roles of the RAS pathway in human genetic disorders. Hum Mutat. 2008;29(8):992–1006.
Humphreys RC, Halpern W. Trail receptors: targets for cancer therapy. In: Khosravi-Far R, White E, editors. Programmed cell death in cancer progression and therapy. Dordrecht: Springer; 2008. p. 127–58.
Logue SE, Martin SJ. Caspase activation cascades in apoptosis. Biochem Soc Trans. 2008;36(1):1–9.
Meulmeester E, Jochemsen AG. p53: a guide to apoptosis. Curr Cancer Drug Targets. 2008;8(2):87–97.
Salvesen GS, Riedl SJ. Caspase mechanisms. In: Khosravi-Far R, White E, editors. Programmed cell death in cancer progression and therapy. Dordrecht: Springer; 2008. p. 13–23.
Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6(3):184–92.
Osaki M, Oshimura MA, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9(6):667–76.
Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23(18):3151–71.
Wu X, Yu J, Yan J, Dai J, Si L, Chi Z, Xu T. PI3K/AKT/mTOR pathway inhibitors inhibit the growth of melanoma cells with mTOR H2189Y mutations in vitro. Cancer Biol Ther. 2018;19(7):584–9.
Wang P, Guan Q, Zhou D, Yu Z, Song Y, Qiu W. miR-21 inhibitors modulate biological functions of gastric cancer cells via PTEN/PI3K/mTOR pathway. DNA Cell Biol. 2018;37(1):38–45.
Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog Neurobiol. 2011;93(2):182–203.
Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35.
Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018;19(9):547–62.
Guo SL, Ye H, Teng Y, Wang YL, Yang G, Li XB, Zhang C, Yang X, Yang ZZ, Yang X. Akt-p53-miR-365-cyclin D1/cdc25A axis contributes to gastric tumorigenesis induced by PTEN deficiency. Nat Commun. 2013;4(1):1–11.
Yang Y, Shao N, Luo G, Li L, Zheng L, Nilsson-Ehle P, Xu N. Mutations of PTEN gene in gliomas correlate to tumor differentiation and short-term survival rate. Anticancer Res. 2010;30(3):981–5.
Tate G, Suzuki T, Nemoto H, Kishimoto K, Hibi K, Mitsuya T. Allelic loss of the PTEN gene and mutation of the TP53 gene in choriocarcinoma arising from gastric adenocarcinoma: analysis of loss of heterozygosity in two male patients with extragonadal choriocarcinoma. Cancer Genet Cytogenet. 2009;193(2):104–8.
Wang Q, Zhao Y, Zhang T, Kou Y. Expression and correlation of survivin and PTEN in gastric cancer. J China Med Univ. 2009;38(4):270–3.
Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27(13):2278.
Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Chem Biol. 2005;17(6):596–603.
Kim D, Dan HC, Park S, Yang L, Liu Q, Kaneko S, Nicosia SV. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005;10(14):975.
Zheng Z, He XY, Li JF, Yu BQ, Chen XH, Ji J, Liu BY. RhoGDI2 confers resistance to 5-fluorouracil in human gastric cancer cells. Oncol Lett. 2013;5(1):255–60.
Lei Z, Tan IB, Das K, Deng N, Zouridis H, Pattison S, Ivanova T. Identification of molecular subtypes of gastric cancer with different responses to PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology. 2013;145(3):554–65.
Wendel HG, Malina A, Zhao Z, Zender L, Kogan SC, Cordon-Cardo C, Lowe SW. Determinants of sensitivity and resistance to rapamycin-chemotherapy drug combinations in vivo. Cancer Res. 2006;66(15):7639–46.
Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28(6):1075.
Murugan AK. mTOR: Role in cancer, metastasis and drug resistance. In: Seminars in cancer biology, vol. 59. Cambridge: Academic Press; 2019. p. 92–111.
Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497–510.
Cohen P. The origins of protein phosphorylation. Nat cell biol. 2002;4(5):E127–30.
Peponi E, Drakos E, Reyes G, Leventaki V, Rassidakis GZ, Medeiros LJ. Activation of mammalian target of rapamycin signaling promotes cell cycle progression and protects cells from apoptosis in mantle cell lymphoma. Am J Pathol. 2006;169(6):2171–80.
Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31(6):342–8.
Wendel HG, de Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428(6980):332–7.
Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17(8):666–81.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101.
Yao C, Liu J, Shao L. Rapamycin inhibits the proliferation and apoptosis of gastric cancer cells by down regulating the expression of survivin. Hepatogastroenterology. 2011;58(107–108):1075–80.
Li Y, Cui J, Zhang CH, Yang DJ, Chen JH, Zan WH, He YL. High-expression of DJ-1 and loss of PTEN associated with tumor metastasis and correlated with poor prognosis of gastric carcinoma. Int J Med Sci. 2013;10(12):1689.
Zhu X, Qin X, Fei M, Hou W, Greshock J, Bachman KE, Qin CY. Loss and reduced expression of PTEN correlate with advanced stage gastric carcinoma. Exp Ther Med. 2013;5(1):57–64.
Yusufu A, Tuerdi R, Redati D, Rehemutula A, Zhao ZL, Wang HJ. Expression and clinical correlation of Survivin and PTEN in gastric cancer patients. Oncol Lett. 2020;20(6):1–1.
Bessede E, Staedel C, Amador LA, Nguyen PH, Chambonnier L, Hatakeyama M, Varon C. Helicobacter pylori generates cells with cancer stem cell properties via epithelial–mesenchymal transition-like changes. Oncogene. 2014;33(32):4123–31.
Zhang B, Zhang X, Jin M, Hu L, Zang M, Qiu W, Guo D. CagA increases DNA methylation and decreases PTEN expression in human gastric cancer. Mol Med Rep. 2019;19(1):309–19.
Acknowledgements
We would like to thank Professor Xiao Yang for providing the SP-A-Cre and PTENfl/fl mouse strains, Dr. Yan Teng and Dr. Shui-long Guo for their helpful discussion and technical support. Dr. Wen-peng Huang provided technical assistance in histopathology. This work was supported by the National Key Project on Drug Development by the Ministry of Science and Technology of China (2009ZX09501-027; 2009ZX09501-034) and the VILLUM FONDEN Foundation of Denmark through the VILLUM Experiment Program.
Author information
Authors and Affiliations
Contributions
C-hZ and CP designed experiments, analyzed the data, and wrote the manuscript. C-hZ and L-lC conducted experiments. WC supported results interpretation and reviewed the manuscript. L-sZ, Z-mZ, LJ, and T-fZ cultured mice and analyzed samples. CP submitted the manuscript. S-qP, J-bG, and CP provided financial support and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, Ch., Peng, Sq., Cui, Ll. et al. Synergistic effects of Rapamycin and Fluorouracil to treat a gastric tumor in a PTEN conditional deletion mouse model. Gastric Cancer 25, 96–106 (2022). https://doi.org/10.1007/s10120-021-01229-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-021-01229-x