Abstract
Gastric cancer is one of the most common malignancy worldwide. In unresectable or metastatic disease, the prognosis is poor and in generally less than a year. HER2 expression remains an important biomarker to lead the addition of trastuzumab to first-line systemic chemotherapy in unresectable or metastatic gastroesophageal adenocarcinoma. To date, a major issue is represented by resistance to trastuzumab developed during treatment, considering the not improved outcomes in this molecular subtype of gastroesophageal adenocarcinoma to other HER2 target strategies. In this review, we summarize the available data on the mechanisms underlying primary and secondary resistance to HER2-targeted therapy and current challenges in the treatment of HER2-positive advanced gastric cancer refractory to trastuzumab. Furthermore, we describe the prognostic value of new non-invasive screening methods, under development novel agents (e.g., HER2 antibody-drug conjugates and bispecific antibodies) and strategies with antitumor activity in early studies.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is the fifth most common cancer worldwide [1]. According to 2018 estimates, GC caused more than 7,00,000 deaths annually, representing the third leading cause of cancer death, mainly due to the advance stage present at the diagnosis [2,3,4,5]. For these patients with advanced, unresectable disease, systemic chemotherapy is the gold standard in the first-line treatment [6, 7]. Despite the improvement observed during the past decades and the steady decline in the incidence of mortality, the prognosis of advanced GC is very poor with a median overall survival (OS) of 10–12 months [8, 9]. The unavailability of accurate diagnostic test for early detection and the absence of valuable prognostic factors, are critical limitations for diagnostic and therapeutic process in the GC treatment. The Cancer Genome Atlas classification identifies four molecular subtypes of GC; the identification of a subgroup of cancer that overexpress human epidermal growth factor 2 (HER2) has allowed major improvements in the treatment [2, 10]. Herein, we report the status of HER2-positive advanced GC treatment, highlighting the mechanisms potentially linked to anti-HER2 drugs resistance. Moreover, new detection methods for HER2 status and the future therapeutic strategies are discussed.
The epidermal growth factor 2 (HER2) pathway
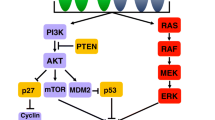
The family of human epidermal growth factor receptor (HER) have been implicated in the development of different tumours and comprises four members: HER 1 (ErbB1), HER 2 (ErbB2), HER 3 (ErbB3) and HER 4 (ErbB4). where ErbB refers to the Erb-b gene responsible for avian erythroblastosis [11]. The protein structures of all HER receptors are composed by an intracellular domain with tyrosine kinase properties, a transmembrane lipophilic domain and a cysteine-rich extracellular ligand-binding domain [12]; they regulate cell differentiation, growth and survival for the development of embryo and adult tissues [13] (Fig. 1). HER receptor family exist as monomers on the cell surface and, once the ligand has bound to the extracellular domain, dimerization of HER proteins took place followed by the transphosphorylation of intracellular domains [13].
HER2 activation. Activation of the HER2 receptor triggers a number of downstream signaling steps through cytoplasm and nucleus, culminating with increased cell growth, survival, and motility. Activation of PI3K/Akt pathway is one of the most studied processes involved with HER2 activation. PI3K converts phosphatidylinositol (4,5)-bisphosphate (PIP2) into phosphatidylinositol (3,4,5)- triphosphate (PIP3). PIP3 acts as a docking site for pleckstrin homology (PH)-containing proteins, such as Akt that becoming phosphorylated Akt activates mTOR and other intracellular pathways resulting in cell proliferation, invasion, and survival. PIP3 in turn is dephosphorylated back to PIP2 by PTEN. PTEN is therefore a negative regulator of PI3K/Akt signaling and functions as a tumor suppressor. The RAS/Raf/ MAPK signaling cascade is also triggered by HER2 activation. Growth Factor Receptor bound Protein 2 (GRB2) binds to the guanine nucleotide exchange factor Son of Sevenless (SOS) that becomes activate and removes guanosine diphosphate (GDP) from inactive RAS. Free RAS can then bind guanosine-5′-triphosphate (GTP) and become active. RAS/GTP binds efficiently to Raf-1 (MAP3K), which becomes activated. Raf-1 can then activate MEK that phosphorylates and activates the extracellular signal-regulated kinase ERK resulting in cell cycle control, differentiation, and migration. Figure created with Biorender.com
In detail, HER2 receptor, whose gene is located on the human chromosome 17 (17q12) and also known as ErbB2, p185 or neu, is a 185 kD (1255 amino acid) transmembrane glycoprotein [14, 15]. As aforementioned, its inappropriate activation, mainly due to overexpression via HER2 gene amplification and other secondary genetic mechanisms [16, 17], is correlated with the development of different malignancies such as gastric, ovarian, breast, pancreatic, colorectal and endometrial cancer [13, 18,19,20]. HER2 has no specific single activating ligand and it has been speculated that its constitutively activated state might occur following the heterodimerization with other family members (HER1 and/or HER3) [21]. HER2 activation leads to the autophosphorylation of tyrosine residues within the cytoplasmic domains and the activation of different signalling pathways, primarily protein kinase C (PKC), phosphatidylinositol-4,5 bisphosphate 3-kinase (PI3K) and the mitogen-activated protein kinase (MAPK) resulting in in cell survival, angiogenesis, and metastasis [13, 18]. Heterodimers containing HER2 provide a more robust signal than stand-alone homodimers or when coupled with other family members, with a significant higher ligand-binding affinity [22]. For instance, the PI3K/Akt downstream pathway, which is considered the leader regulator of cell growth, is generated by HER2–HER3 heterodimer [21, 22]. HER2 dimerization leads to cell-cycle progression also supporting the fast processing of cell-cycle inhibitor p27 [21]. Lastly, HER2 can be triggered via the dimerization with other membrane receptors including the insulin growth factor-1 (IGF1) [23].
HER2 amplification/over-expression
Amplification or over-expression of HER2 has demonstrated an crucial role in the development and progression of certain aggressive cancers (e.g., breast and GC) [24,25,26]. Recently, the protein has become a therapy biomarker and target for patient with GC, in which HER2 overexpression ranges from 6 to 30% of cases [2].
HER2 status assessment is performed on a biopsy tissue sample from the tumor using immunohistochemistry (IHC) that measures the amount of HER2 protein expressed by cancer cells reported on a scale from 0 to 3+ (negative 0− 1+, equivocal 2+ or positive 3+) and fluorescence in situ hybridization (FISH) that evaluate the number of HER2 gene copies and a dichotomous result are reported (negative or positive) FISH is currently recommended in the case of equivocal HER2 score. The correlation between IHC and FISH is elevated, although intra-tumoral heterogeneity, technical errors or other genetic factors, can lead to inconsistent results [27, 28].
HER2-positive tumors usually have a higher tumor grade growing and spreading more rapidly than cancers with a normal expression of HER2. HER2 overexpression was found to be a negative prognostic factor in GC in some studies, even though its role remains still uncertain [29,30,31,32].
Anti-HER2 agents in first-line positive advanced gastric cancer
Trastuzumab
Trastuzumab is a humanized monoclonal antibody targeting HER2, that exerts its antitumor-mediated response causing the internalization and downregulation of HER2 and inhibiting cancer cell proliferation [33, 34]. First, it was approved for the treatment of HER2 positive breast cancer; subsequently, its use was authorized for advanced GC, based on the result of the ToGA trial [35, 36]. In this phase III trial, patients with advanced GC or gastroesophageal junction adenocarcinoma with HER2 overexpression (ICH3+ or FISH+) were randomly assigned to receive first-line chemotherapy (cisplatin and fluoropyrimidine) with or without trastuzumab. Trastuzumab-containing regimen yielded an increased median overall survival (OS), (13.8 vs 11.1 months; p = 0.0046), median progression-free survival (PFS) (6.7 vs 5.5 months p = 0.0017) and overall response rate (ORR) (47 vs 35%, p = 0.0017). The safety profile was similar in the two groups, and no differences were observed in cardiac-related events. Post hoc analysis showed null or lower benefit from adding trastuzumab to chemotherapy in patients with IHC 0 or 1+ and FISH+, compared to patients with ICH2 or 3+ and FISH+ [2, 36, 37]. Henceforth, similar efficacy was also assessed by combining trastuzumab with other chemotherapeutic regimens. In particular, three phase II studies evaluated the combination of trastuzumab with capecitabine and oxaliplatin reporting a median OS of 13.8–21.0 months, a median PFS of 7.1–9.8 months, and an ORR of 46.7–67.3% [38,39,40]. In addition, a meta-analysis proved that capecitabine or 5FU can be replaced by S−1 and cisplatin by oxaliplatin [38, 40,41,42,43]. Intriguingly, trastuzumab seems to elicit T cell response; therefore, its association with immune checkpoint inhibitors has earned attraction in the last few years [44,45,46].
Lapatinib
Lapatinib is a dual tyrosine kinase inhibitor which affects both HER2 and epidermal growth factor receptor (EGFR) [47]. It was evaluated in a phase III trial (TRIO-013/LOGiC) for patients with HER2-positive GC in combination with capecitabine and oxaliplatin failing to achieve its primary endpoint of OS against chemotherapy alone (12.2 and 10.5 months, respectively) [48].
Pertuzumab
Pertuzumab is a recombinant humanized monoclonal antibody that inhibits the dimerization of HER2 with other HER receptors, which prevents signaling promotion and, thus, cell growth and proliferation. The phase III JACOB trial examined the effect of the addition of pertuzumab to trastuzumab and chemotherapy for patients with HER2-positive advanced GC [49]. No significant improvement regarding its primary endpoint of OS was observed (14.2 vs 17.5 months, HR 0.84, 95% C.I. 0.71–1.00, p = 0.057), despite the benefit in terms of PFS (8.5 vs 7.0 months, HR 0.73, 95% C.I. 0.86, p =0.0001). These data support the intrinsic differences in the HER2 biology between breast and GC cells in driving disease progression, probably due to the heterogeneous pattern observed in GC [50, 51] (Table 1).
Anti-HER2 agents in second line advance positive gastric cancer
Lapatinib
The survival benefit of lapatinib combined with paclitaxel in second-line setting to test its efficacy in restoring trastuzumab sensitivity was evaluated in the phase III trial TyTAN. [52, 53] No OS benefit, PFS or time to progression improvement was recorded for lapatinib in the whole cohort. In a subgroup analysis of data from patients with baseline IHC3+ tumors, OS (14 vs 7.6 months; HR 0.59, 95% C.I. 0.37–0.93, p = 0.02) and PFS (5.6 vs 4.2 months; HR 0.54 95% C.I. 0.33–0.90, p = 0.010) were significantly higher in lapatinib arm [53]. These results suggest that lapatinib would be beneficial in patients with HER2 IHC 3+ GC, but this approach is not recommended in clinical practice. Noteworthy, in this study, 35% of the patients were IHC0/1+ and, to date, this would be considered as HER2 negative [53].
Trastuzumab emtansine
Trastuzumab emtansine (T-DM1) is a monoclonal antibody conjugate of trastuzumab linked to the tubulin inhibitor emtansine. Based on promising results about the antitumor activity of T-DM1 in HER2-positive GC cells, even in trastuzumab-resistant tumors [54], the phase II/III trial (GASBY) compared T-DM1 with the physician’s choice in the second-line setting. No improvements in terms of OS and PFS with the experimental arm were observed [55]. Recently, a phase I/II trial evaluated T-DM1 plus capecitabine in first-line treatment of HER2-positive advanced GC was stopped for treatment failure (NCT01702558). Among the reasons for study failure, the evaluation of HER2 status through archival tissue specimens should be primarily considered. The loss of HER2 expression is common among patients with HER2-positive GC receiving HER2-targeted therapies and might explain the failed response to T-DM1. Interestingly, several acquired resistance processes have been recently explored and will be further described below.
Trastuzumab beyond progression
Trastuzumab is currently the only anti-HER2 agent capable of demonstrating efficacy in a randomized phase III study. In patients with HER2-positive breast cancer, the use of trastuzumab beyond progression is an established treatment strategy that has been shown to prolong survival outcomes [56]. Recently, this approach has also been proposed in HER2-positive advanced GC by several studies; however, the results are still conflicting. Particularly, in the phase II randomized clinical trial conducted by the West Japan Oncology Group (WJOG7112G/T-ACT), trastuzumab plus paclitaxel showed no benefit over paclitaxel alone in GC patients resistant to first-line trastuzumab-based therapy [57,58,59,60,61].
In a retrospective analysis, maintenance with trastuzumab in the second-line setting after first-line trastuzumab-based therapy, recorded a longer median PFS (4.4 vs 2.3 months) and OS (12.6 vs 6.1 months) compared with chemotherapy alone [57, 58].
Conversely, other studies demonstrated no benefit of trastuzumab beyond progression, despite an increased PFS (4.6 vs 2.9 months) in patients not exposed to anti-HER2 agents for a period longer than 30 days [59,60,61] (Table 2).
Based on these results, a resensitization to trastuzumab after a treatment-free interval, could reduce selective pressure on HER2-positive clones [62]. In this landscape, the identification of patients who would benefit from the continuation of HER2 blockage beyond progression could be determinant for this strategy.
Therapeutic perspective in HER2-positive gastric cancer
ZW25
ZW25 is a bispecific antibody that simultaneously binds to two HER2 epitopes: ECD4, the trastuzumab-binding domain, and ECD2, the pertuzumab-binding domain [63]. Preclinical studies suggested that ZW25 has strong antitumor activity at a range of HER2 expression levels and may more effectively silence HER2 signaling than trastuzumab or pertuzumab and stimulates the immune system. It was tested in a phase I basket trial showing encouraging efficacy in pretreated patients with HER2-positive gastroesophageal cancer. ORR and disease control rate was 44 and 56%, respectively [64]. Adverse events (AEs) were all grade 1–2 except for one patient who experienced reversible grade 3 hypophosphatemia, arthralgia, and fatigue. Based on these results, ZW25 has been granted a fast-track designation by the Food and Drug Administration (FDA) for the treatment of patients with HER2-overexpressing gastroesophageal adenocarcinoma to be used in combination with standard of care chemotherapy. Currently, a trial to assess the safety, tolerability and preliminary antitumor activity of ZW25 in combination with tislelizumab (a humanized monoclonal antibody directed against PD-1) and chemotherapy in patients with HER2-positive gastric/gastroesophageal junction adenocarcinoma is ongoing (NCT04276493).
Margetuximab
Margetuximab is a next-generation Fc-modified anti-HER2 monoclonal antibody that binds with elevated affinity CD16A, an Fc receptor important for antibody dependent cell-mediated cytotoxicity (ADCC) against tumor cells [65]. A phase I study, including 20 patients with GC, evaluated the toxicity profile, maximum tolerated dose, pharmacokinetics features, and antitumor activity of margetuximab in patients with HER2-overexpressing carcinomas [66].
Over half [45/66 (68%)] of patients received at least one prior anti-HER2 therapy in the metastatic setting. Common toxicities were primarily ≤ grade 2; grade 3/4 AEs attributed to margetuximab were infrequent and included: increased lipase, blood amylase, blood alkaline phosphatase lymphocyte decreased, and infusion-related reaction (IRR), including cytokine release syndrome [66].
Despite the heavily pre-treated population, margetuximab demonstrated evidence of clinical activity with an ORR of 12%. The effect of single-agent margetuximab in the population studied suggests potential for antitumor activity after progression or following other anti-HER2 regimens. Moreover, in a single-arm phase Ib/II trial, margetuximab has been used in combination to pembrolizumab in HER2-positive advanced GC patients, as second-line therapy [67]. The preliminary results have demonstrated acceptable toxicities with serious AEs (grade ≥3) in about 9% of patients, including autoimmune hepatitis. The response rate was ~ 20% in the 92 evaluable patients. Noteworthy, all responses have been reported in patients with HER2 ICH 3+ and the responses were more frequent PD-L1-positive tumors. Preliminary data of median PFS of 3 months and median OS of 13 months were reported [68].
The ongoing phase II/III randomized open-label trial (MAHOGANY) for treatment of patients with HER2-positive GC was designed to determine the efficacy of margetuximab combined with an anti-PD-1 monoclonal antibody, INCMGA00012 (also known as MGA012) (Cohort A) and margetuximab combined with INCMGA00012 or MGD013 (anti-PD-1/anti-LAG-3 dual-affinity re-targeting protein) and chemotherapy compared to trastuzumab combined with chemotherapy (Cohort B) [69].
Trastuzumab deruxtecan
Trastuzumab deruxtecan (T-DXd) is an antibody-drug conjugate composed of an anti-HER2 antibody, a cleavable tetrapeptide-based linker, and a cytotoxic topoisomerase I inhibitor [70]. The FDA approved as fast-track designation, trastuzumab deruxtecan for female patients with HER2-positive, unresectable and/or metastatic breast cancer [71, 72]. In two phase I trials, trastuzumab deruxtecan was administered in pretreated GC patients, recording ORR about 43%. Serious treatment AEs (mainly grade ≥3 myelosuppression) were reported in the 25% of patients [73,74,75]. A randomized phase II trial (DESTINY-Gastric01) evaluated efficacy and safety of trastuzumab deruxtecan in advanced HER2-positive GC [76]. Patients who progressed during treatment (two or more previous regimens including trastuzumab) have been enrolled to receive trastuzumab deruxtecan or the physician’s choice recording an ORR of 51.3 and 14.3% in two groups, respectively. The safety profile was generally manageable, and the common AEs were hematologic or gastrointestinal. Based on DESTINY-Gastric01 trial, FDA has recently approved Trastuzumab deruxtecan for the treatment of adult patients with HER2-positive locally advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma who have received a prior trastuzumab-based regimen
Trastuzumab deruxtecan is effective not only against tumor cells positive for HER2 protein but also, in the presence of HER2-positive cells, against those negative for such expression [77]. This “bystander killing effect” seems due to the internalization of trastuzumab deruxtecan by HER2-positive cells, the release of DXd into the cytoplasm and the subsequent transfer of DXd into adjacent HER2-negative cells [78]. Indeed, trastuzumab deruxtecan has proven effectiveness against tumors that express HER2 but are negative for HER2 amplifications [77]. Moreover, the efficacy of trastuzumab deruxtecan is independent to the absence or presence of other gene alterations that can active alternative pathways, suggesting the possibility of overcoming trastuzumab resistance [79]. Therefore, the action of trastuzumab deruxtecan could be significant in cases with absent homogeneity of HER2 amplification and expression.
A phase Ib/II trial (DESTINY-Gastric03) is testing safety and efficacy of trastuzumab deruxtecan alone or in combination with chemotherapy and/or immunotherapy in HER2-positive advanced or metastatic gastric/gastroesophageal junction adenocarcinoma patients (NCT004379596).
Pan-HER tyrosine-kinase inhibitors
Considering the suggested antitumor efficacy of pan-HER blockade , several pan HER2-targeted tyrosine-kinase inhibitors (TKIs) have been evaluated in clinical trials for GC treatment [80].
Afatinib is an oral kinase inhibitor that irreversibly blocks EGFR, HER2, HER3, and HER4 [81]. It is being investigated for breast cancer as well as other EGFR/HER2-driven cancers, including HER2 positive gastrointestinal tumors [82]. It was evaluated in a phase II study in patients with esophagogastric cancer previously treated with trastuzumab, providing a moderate therapeutic benefit with an ORR of 10% [83]. A phase II study with afatinib and paclitaxel in patients with HER2-positive GC progressed to trastuzumab and chemotherapy is currently ongoing (NCT02501603; NCT01522768).
Poziotinib is another TKI that binds irreversibly to the active site of the tyrosine kinase domain and blocks signal transduction by EGFR, HER2, and HER4. It has been tested in a phase I/II study, in which patients with HER2-positive advanced GC, previously treated with one line of chemotherapy regardless trastuzumab exposure, were enrolled to receive poziotinib with trastuzumab and paclitaxel. A median PFS of 13 weeks and a median OS of 29.5 weeks was reported [84].
Dacomitinib, an irreversible pan-HER inhibitor, has been evaluated in monotherapy, in a multicenter phase II study showing anti-tumoral activity in HER2-positive GC. Patients enrolled had received from one to more than three prior chemotherapy regimens and a median PFS and OS of 2.1 and 7.1 months has been reached, respectively [85].
Immune checkpoint inhibitors
Blocking immune checkpoint, especially programmed cell death-1 (PD-1) and its ligand (PD L1 or B7-H1), has proven efficacy in several solid cancers, and seems to become a potential option in gastric cancer treatment [86]. The efficacy of immunotherapy in GC has been tested in several studies, but few data on the efficacy in the specific setting of HER2-positive GC have been published.
The combination of pembrolizumab plus trastuzumab, fluoropyrimidines and oxaliplatin has been investigated as first-line therapy in a small phase II cohort of patients in HER2-positive advanced GC. A median PFS of 11.3 months, with 67% 6 months of PFS and an ORR of 87% has been observed [45].
The phase III KEYNOTE-811 trial to confirm these results, comparing chemotherapy plus trastuzumab with or without pembrolizumab, is currently ongoing (NCT03615326). Moreover, the phase 2 trial (INTEGA) is assessing the efficacy of two experimental first-line treatment strategies in advanced or metastatic esophagogastric adenocarcinoma: chemo-free immunotherapy with trastuzumab, nivolumab and ipilimumab or addition of nivolumab to the standard regimen (FOLFOX chemotherapy and trastuzumab) (NCT03409848).
Moreover, an exploratory subgroup analysis of HER2-positive patients of phase III ATTRACTION-2 trial, assessed a significant longer OS in patients receiving nivolumab with history of trastuzumab use vs placebo (8.3 vs 3.1 months; HR: 0.38, 95% C.I. 0.22–0.66, p= 0.0006) [87].
Several clinical trials are evaluating the safety and efficacy of different immune checkpoint inhibitors in patients with advanced G/GEJ cancer. The combination of anti-HER2 and immunotherapeutic agents seems to be a promising strategy [88,89,90,91,92].
These encouraging results have raised again the interest in developing and assessing strategies acting on HER2 in GC. Indeed, a decade after the publication of the TOGA trial, no other study had confirmed the validity of the strategy either in the first or in the second line. These innovative molecules in the GC treatment seem to have the potential of synergistic effect on innate and acquired immunity, combining immunotherapy with an engineered intervention aimed at optimizing precision medicine (Table 3).
Mechanism of resistance to anti-HER2 agents
HER2 positivity accounts for around 15% of GC, although its expression is quite heterogeneous compared with breast cancer (from 26 to 79% in IHC), which could negatively affect response to anti-HER2 targeted therapy [37, 93, 94] (Fig. 2a). Indeed, although the inhibition of HER2 has dramatically influenced the overall outcomes of HER2-positive GC patients, over 75% of them develop disease progression within 12 months. Of note, several potential mechanisms may give rise to primary and secondary resistance to HER2 blockage in breast cancer: impaired access of trastuzumab to HER2 by expression of an extracellular domain-truncated form of HER2 or overexpression of MUC4; activation of downstream signaling pathways (PI3K/AKT, MAPK, MEK and mTOR); loss of downstream controllers (PTEN and p27); alternative signaling from the insulin-like growth factor-1 receptor, or mesenchymal–epithelial transition (MET). However, all molecular mechanisms underlying resistance to HER2-targeted therapy in GC are not yet fully characterized, although some of them are shared with breast cancer.
General mechanisms of resistance to trastuzumab: presence of upregulation of HER2 downstream signaling pathways. a HER2 expression and/or amplification is highly heterogeneous in GC and this heterogeneity may negatively affect the response to HER2 blockage strategies leading to primary resistance. b Genomic aberrations in the PI3K pathway produce constitutive activation of the pathway, which will signal downstream to the nucleus regardless of trastuzumab binding to HER2. This is the case with activating mutations of PIK3R1 and PIK3CA, encoding genes for PI3K p85α and p110α, respectively. c PTEN is a tumor suppressor. Trastuzumab binding stabilizes and activates PTEN and consequently down-regulates the PI3K/Akt signaling pathway. When PTEN function is lost, PI3K remains constitutively active regardless of binding of trastuzumab to HER2 causing unresponsiveness to trastuzumab treatment. d Trastuzumab-induced growth inhibition in HER2-overexpressing cells can be compensated for by increased of other pathways, resulting in resistance to trastuzumab. c-Met is frequently co-expressed with HER2 in cell lines and its amplification or an increase of its ligand (HGF), contribute to trastuzumab resistance through sustained Akt activation. Figure created with Biorender.com
Tumor heterogeneity—primary resistance
GC is a highly heterogeneous malignancy with a complex genomic landscape of molecular alterations. In breast cancer cells, the membranous distribution of the antibody is generally circumferential and complete staining for HER2 is required for the tumor to be classified as HER2 positive, whereas in GC, it is prevalently basolateral and incomplete, associated with intra-tumoral heterogeneity which provides a possible explanation for the differences in efficacy of the same HER2-targeted therapies between patients with GC and those with breast cancer. Notably, HER2 expression is highly discrepant between primary and metastatic disease [95, 96]. In the GASTHER1 study, 5.7% initial HER2-negative patients on primary tumor resulted HER2-positive on metastatic sites, reaching 17% discordance for liver lesions [97].
Co-existing oncogenic alterations—primary resistance
Oncogenic alterations such as point mutations or amplification have been reported, leading to the activation of downstream pathways and hampering the inhibitory effect of HER2-directed agents [66]. Those genetic alterations may be primed as negative predictors of trastuzumab benefit and, potentially, exploited as therapeutic co-targets. PI3KCA-activating mutations (Fig. 2b) and/or PTEN loss (Fig. 2c) may cause constitutive activation of the AKT–mTOR pathway, and the constitutive/aberrant activation of this signaling cascade may result in an inefficiency HER2 inhibition [98,99,100]. Deguchi et al. analyzed the occurrence of HER2 expression and PI3K mutation or PTEN loss in 264 GC patients, reporting 34.5% of HER2-postive patients with PTEN loss. No responses were observed in patients with PTEN loss treated with trastuzumab [98].
Hyperactivation of the hepatocyte growth factor (HGF) or the amplification of MET may be involved in the primary resistance in GC (Fig. 2d) [101,102,103]. Takahashi et al. showed that high-serum HGF was associated with poor response during HER2 blockage [98]. Furthermore, HER2 and HER1 may co-amplify in about 7% of GCs, according to the TCGA database report [104]. Preclinical studies showed that those cases may be resistant to upfront trastuzumab, while dual inhibitors might be beneficial in obtaining a more complete growth inhibition.
A multicenter, prospective, case–control study including 37 HER2-positive GC patients tested the negative predictive impact of a panel (AMNESIA) evaluating EGFR/MET/KRAS/PI3K/PTEN mutations and EGFR/MET/KRAS amplifications [105]. AMNESIA panel alterations were significantly more frequent in resistant (11 out of 20; 55%) as compared to sensitive (0% of 17) patients (p < 0.001). Furthermore, GC patients without panel alterations had a significantly longer median PFS (5.2 vs 2.6 months, HR: 0.34, 95% CI: 0.07–0.48, p = 0.001) and OS (16.1 vs 7.6 months, HR: 0.38, 95% CI: 0.09–0.75, p = 0.015) compared to the GC patients with panel positive tumors. The predictive accuracy of AMNESIA panel and HER2 IHC was 76 and 65%, respectively, while the predictive accuracy of the combined evaluation of AMNESIA panel and HER2 IHC was 84%. However, the retrospective nature of the study, the lack of a control arm (without trastuzumab), the elevated cost of the molecular screening, and the impossibility of detecting rare mutations may limit the current application in the clinical practice. A more extended, prospectively validated database called AMNESIA Global is currently ongoing.
Activation of alternative pathways—acquired resistance
HER2 acquired mutations src-induced activation of the MAP/ERK downstream pathway, NRF2 expression, FGFR amplification or HER3 overexpression may all contribute to the development of secondary resistance to trastuzumab [106,107,108,109,110].
Combination regimens involving HER2-targeted agents and those targeting IGF1R, PI3K, SRC and other proteins might overcome resistance to already approved HER2-targeted agents.
Loss of HER2 positivity—acquired resistance
Several authors have addressed the issue of the loss of the target during the upfront treatment, showing that a substantial proportion of patients may have a complete decline in surface HER2 expression after trastuzumab exposure [111]. The disappearance of the target is particularly frequent in the HER2-positive tumors classified as IHC2+ and FISH+ that may justify the insufficient HER2-inhibitor activity. Re-evaluation of HER2 status at disease recurrence or disease progression is needed to determine the appropriate use of HER2-targeted therapies, although is not considered mandatory in clinical practice.
Micro RNAs—acquired resistance
Upregulation of several microRNAs may regulate genes involved in the HER2 signaling pathway or HER3 at the post-transcriptional level and may be involved in acquired resistance to HER2-targeted therapies [112, 113].
Epithelial-to-mesenchymal transition—acquired resistance
Preclinical evidence suggest that MET may be involved in HER2-inhibition secondary resistance [114, 115]. Particularly, Jialong et al. showed a mesenchymal phenotype, increased migration, and invasive capacities in HER2-positive GC cell lines.
New screening techniques
The development of newer and performing diagnostic, prognostic and disease monitoring tools are crucial for the improvement of the clinical outcome of GC patients [4]. Tissue samples were the main sources for evaluating tumor-associated genetic alterations in these patients, but the invasive nature and the inability to reflect tumor heterogeneity are crucial limitations [36].
Other methods, such as circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), extracellular domain (ECD) of HER2 and new imaging agents [i.e., zirconium 89 (89Zr) trastuzumab PET], have demonstrated to have prognostic value in several types of cancer, including GC, or used as non-invasive tool to monitor disease progression throughout treatment [116,117,118,119].
A prognostic role for the enumeration of CTC in numerous cancers, including GC has been highlighted [120,121,122]. CTCs may represent the genetic compositions of both primary and metastatic tumors, and the real-time assessment of prognostic and therapeutic biomarkers on CTCs may be useful to improve the clinical outcomes and effecting on targeted cancer therapy [116, 117]. Indeed, Nevis et al., have demonstrated that the HER2 amplification in CTCs, evaluated using FISH, was strongly concordant with tissue amplification, considering their application as a potential alternative to tumor biopsy [123].
Nonetheless, this accordance was not confirmed in another study where13 out to 50 patients with GC considered HER2 negative, had a benefit to trastuzumab therapy similar to patients with GC HER2-positive tumor in preliminary clinical data [124].
Circulating tumor DNA is tumor-derived fragmented DNA in the bloodstream not associated with cells [125]. Originating from primary tumor cells, CTCs and/or distant metastasis, ctDNA give a broad cross-section of the disease offering information on methylation status, genetic alterations as mutations, amplifications, rearrangements, copy number variation (CNV) [126]. ctDNA represents only a small percentage of the cell-free circulating DNA (cfDNA), which is increased considerably in late-stage disease [127]. However, ctDNA can be detected in the plasma of cancer patients even in the early stages of disease [128, 129]. Circulating tumor DNA analysis refined the liquid biopsy to identify tumor molecular traces circulating and may give deeper insight into the cancer heterogeneity, early biomarker detection, therapeutic target detection, real-time evaluation of treatment response and possible resistance and prognosis.
Initially, a concordance rate of HER2 expression between the ctDNA and tumor tissue around 60% has been observed [130], which became up to 90% with the use of modern techniques (i.e., digital droplet PCR, next-generation sequencing) [131, 132]. The difference between plasma and tumor in HER2 amplification could be explained at least in part, by the high heterogeneity of HER2 expression in GC cells but, if confirmed the validity of ctDNA, it could be used as alternative HER2 screening method. Moreover, recent data suggest that ctDNA could be a complementary tool to predict response to anti-HER2 treatment and that their dynamic evaluation could be a surrogate marker of treatment efficacy, although there is still reliable data and further confirmations are needed [132,133,134].
The extracellular domain of HER2, quantifiable in the serum, seems to be another valid alternative to tissue biopsy. A significant relationship between serum concentrations of HER2 ECD and tissue levels of HER2 protein was found in patients with GC [135]. Moreover, HER2 ECD level could become a predictive marker of response to anti-HER2 therapies as has been suggested by several studies in breast cancer [136, 137].
Discordant HER2 expression within a single cancer or between different sites of cancer can introduce sampling error and confound treatment decision-making. First tried in breast cancer and more recently in GC, positron emission tomography (PET), using the radiolabeled zirconium 89 [89Zr] trastuzumab, has shown several advantages over biopsy-based methods as it can noninvasively assess variation in level of HER2 in both the primary tumor and all sites of metastases simultaneously [138]. [89Zr] trastuzumab PET directly assesses the availability of HER2 to be bound to trastuzumab; thus, it is potentially a more reliable predictor of response to trastuzumab therapy. In addition, [89Zr] trastuzumab PET has the ability to assess intra-patient heterogeneity of HER2 tumor expression and considering its not invasiveness [89Zr] trastuzumab PET can be repeated during therapy to assess response [138,139,140].
Recently, the organoid technology has been emerged to overcome the issues related to the widespread histological and molecular heterogenicity that characterize GC as well as other tumors as well as the limitations related to cancer cell lines and patient-derived xenografts (PDX), as reviewed in [141].
Briefly, long-term organoid cultures can be established from several primary tumors (e.g., stomach, breast, colon cancer tissues) and collection of patient-derived tumors are generated and biobanked [141]. These organoid models were used to investigate molecular features in different subtypes and patient treatment response. For instance, a library with more than 100 breast cancers (primary and metastatic tumors) organoid lines has been already built as well as a patient-derived GC organoid library with numerous histological and genetic subtypes [142, 143].
Moreover, the development of single-cell profiling, that allow to study tumors (and the related complex microenvironment) at the resolution of individual cells, could have a profound impact on clinical decisions [144]. Indeed, single-cell sequencing can reveal clonal repopulation, factors related to acquired drug resistance and dynamic changes in tumor-associated microenvironment. To note, Wang et al. have demonstrated that the use of high-throughput single-cell profiling allows to individuate target specific cell population (i.e., intra-tumoral immunosuppressive myeloid cell), targetable by tyrosine kinase inhibitor, that restores the vulnerability to checkpoint blockade immunotherapy, overcoming resistance [145].
Conclusions
Unfortunately, a large portion of patients with GC are initially diagnosed with unresectable or metastatic disease and systemic treatments have led to modest improvement in overall survival when compared to BSC alone. The identification of tumor with HER2 overexpression in metastatic CG patients remains significant to improve treatment outcomes. However, to enable progress beyond currently approved therapies in this molecular subset will require composite testing strategies to properly identify tumoral heterogeneity underlying trastuzumab resistance. Several clinical trials evaluating novel anti-HER2 approaches are ongoing and the introduction of new screening methods, such as circulating tumor cells, circulating tumor DNA, extracellular domain of HER2 and new imaging agents, could deeply improve their therapeutic impact in GC.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86.
Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19(9):1523–9.
Rugge M, Fassan M, Graham DY. Epidemiology of gastric cancer. Gastric Cancer Princ Pract. 2015. p. 23–34.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–64.
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–9.
Fontana E, Smyth EC. Novel targets in the treatment of advanced gastric cancer: a perspective review. Ther Adv Med Oncol. 2016;8(2):113–25.
Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-49.
Zaanan A, Bouché O, Benhaim L, Buecher B, Chapelle N, Dubreuil O, et al. Gastric cancer: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO. Dig Liver Dis. 2018;50(8):768–79.
Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9.
Riese DJ, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. BioEssays. 1998;20(1):41–8.
Van Der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337.
Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:1–9.
Padhy LC, Shih C, Cowing D, Finkelstein R, Weinberg RA. Identification of a phosphoprotein specifically induced by the transforming DNA of rat neuroblastomas. Cell. 1982;28:865–71.
Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, et al. The neu oncogene: an erb-B-related gene encoding a 1,85,000-Mr tumour antigen. Nature. 1984;312:513–6.
Lee JW, Soung YH, Seo SH, Kim SY, Park CH, Wang YP, et al. Somatic mutations of ERBB2 kinase domain in gastric, colorectal, and breast carcinomas. Clin Cancer Res. 2006;12:57–61.
Hollywood DP, Hurst HC. A novel transcription factor, OB2-1, is required for overexpression of the proto-oncogene c-erbB-2 in mammary tumour lines. EMBO J. 1993;12:2369–75.
Olayioye MA. Intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res. 2001;3:385–9.
Neve RM, Lane HA, Hynes NE. The role of overexpressed HER2 in transformation. Ann Oncol. 2001;12:S9–13.
Ménard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22(42):6570–8.
Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26(45):6469–87.
Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–16.
Nahta R, Yuan LXH, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–28.
Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005;353:1652–4.
Meza-Junco J, Au HJ, Sawyer MB. Critical appraisal of trastuzumab in treatment of advanced stomach cancer. Cancer Manag Res. 2011;3:57–64.
Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25(5):637–50.
Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology. 2011;59:832–40.
HER2 Testing in Gastric and Gastroesophageal Adenocarcinoma—: AJSP: Reviews and Reports [Internet]. [cited 2020 Sep 9]. Available from: https://journals.lww.com/pathologycasereviews/Abstract/2019/07000/HER2_Testing_in_Gastric_and_Gastroesophageal.9.aspx.
Jørgensen JT, Hersom M. HER2 as a prognostic marker in gastric cancer -a systematic analysis of data from the literature. J Cancer. 2012;3:137–44.
Allgayer H, Babic R, Gruetzner KU, Tarabichi A, Schildberg FW, Heiss MM. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol. 2000;18:2201–9.
Yonemura Y, Tanaka M, Sasaki T, Fushida S, Kimura H, Ohoyama S. Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res. 1991;51:1034–8.
Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIα gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–8.
Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007; p. 118–45.
Drebin JA, Stern DF, Link VC, Weinberg RA, Greene MI. Monoclonal antibodies identify a cell-surface antigen associated with an activated cellular oncogene. Nature. 1984;312:545–8.
Brufsky A. Trastuzumab-based therapy for patients with HER2-positive breast cancer: from early scientific development to foundation of care. Am J Clin Oncol Cancer Clin Trials. 2010;33(2):186–95.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Hofmann M, Stoss O, Shi D, Büttner R, Van De Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology. 2008;52:797–805.
Ryu MH, Yoo C, Kim JG, Ryoo BY, Park YS, Park SR, et al. Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer. 2015;51:482–8.
Rivera F, Romero C, Jimenez-Fonseca P, Izquierdo-Manuel M, Salud A, Martínez E, et al. Phase II study to evaluate the efficacy of Trastuzumab in combination with Capecitabine and Oxaliplatin in first-line treatment of HER2-positive advanced gastric cancer: HERXO trial. Cancer Chemother Pharmacol. 2019;83:1175–81.
Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer. 2016;16(1):68.
Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer. 2014;110:1163–8.
Takahari D, Chin K, Ishizuka N, Takashima A, Minashi K, Kadowaki S, et al. Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-naïve, HER2-positive advanced gastric cancer. Gastric Cancer. 2019;22:1238–46.
ter Veer E, Creemers A, de Waal L, van Oijen MGH, van Laarhoven HWM. Comparing cytotoxic backbones for first-line trastuzumab-containing regimens in human epidermal growth factor receptor 2-positive advanced oesophagogastric cancer: A meta-analysis. Int J Cancer. 2018;143:438–48.
Chaganty BKR, Lu Y, Qiu S, Somanchi SS, Lee DA, Fan Z. Trastuzumab upregulates expression of HLA-ABC and T cell costimulatory molecules through engagement of natural killer cells and stimulation of IFNγ secretion. Oncoimmunology. 2016;5(4):e1100790.
Janjigian YY, Chou JF, Simmons M, Momtaz P, Sanchez-Vega F, Shcherba M, et al. First-line pembrolizumab (P), trastuzumab (T), capecitabine (C) and oxaliplatin (O) in HER2-positive metastatic esophagogastric adenocarcinoma (mEGA). J Clin Oncol. 2019;37:62–62.
Chung HC, Bang Y-J, Fuchs CS, Qin S-K, Satoh T, Shitara K, et al. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Futur Oncol. 2020;17(5):491–501.
Xia W, Gerard CM, Liu L, Baudson NM, Ory TL, Spector NL. Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24:6213–21.
Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—a randomized phase III trial. J Clin Oncol. 2016;34:443–51.
Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372–84.
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant Pertuzumab and Trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–31.
Baselga J, Cortés J, Kim S-B, Im S-A, Hegg R, Im Y-H, et al. Pertuzumab plus Trastuzumab plus Docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19.
Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–19.
Satoh T, Doi T, Ohtsu A, Tsuji A, Omuro Y, Mukaiyama A, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTA—a randomized, phase III study. J Clin Oncol. 2014;32:2039–49.
Barok M, Tanner M, Köninki K, Isola J. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett. 2011;306:171–9.
Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18:640–53.
Von Minckwitz G, Du Bois A, Schmidt M, Maass N, Cufer T, De Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A German Breast Group 26/Breast International Group 03–05 study. J Clin Oncol. 2009;27:1999–2006.
Palle J, Tougeron D, Pozet A, Soularue E, Artru P, Leroy F, et al. Trastuzumab beyond progression in patients with HER2-positive advanced gastric adenocarcinoma: a multicenter AGEO study. Oncotarget. 2017;8:101383–93.
Narita Y, Kadowaki S, Masuishi T, Taniguchi H, Takahari D, Ura T, et al. Correlation between human epidermal growth factor receptor 2 expression level and efficacy of trastuzumab beyond progression in metastatic gastric cancer. Oncol Lett. 2017;14:2545–51.
Li Q, Jiang H, Li H, Xu R, Shen L, Yu Y, et al. Efficacy of trastuzumab beyond progression in HER2 positive advanced gastric cancer: A multicenter prospective observational cohort study. Oncotarget. 2016;7:50656–65.
Horita Y, Nishino M, Sugimoto S, Kida A, Mizukami A, Yano M, et al. Phase II clinical trial of second-line weekly paclitaxel plus trastuzumab for patients with HER2-positive metastatic gastric cancer. Anticancer Drugs. 2019;30:98–104.
Makiyama A, Sagara K, Kawada J, Kashiwada T, Hosokawa A, Horie Y, et al. A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT). J Clin Oncol. 2018;36:4011–4011.
Kijima T, Arigami T, Uenosono Y, Hiraki T, Yanagita S, Matsushita D, et al. Comparison of HER2 Status before and after Trastuzumab-based Chemotherapy in patients with advanced gastric cancer. Anticancer Res. 2020;40:75–80.
ZW25 Effective in HER2-positive cancers. Cancer Discov. 2019; p. 8.
Meric-Bernstam F, Beeram M, Mayordomo JI, Hanna DL, Ajani JA, Blum Murphy MA, et al. Single agent activity of ZW25, a HER2-targeted bispecific antibody, in heavily pretreated HER2-expressing cancers. J Clin Oncol. 2018;36:2500–2500.
Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcγ receptors. Cancer Res. 2007;67:8882–90.
Kim J, Fox C, Peng S, Pusung M, Pectasides E, Matthee E, et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J Clin Invest. 2014;124:5145–58.
Catenacci DVT, Kang YK, Park H, Uronis HE, Lee KW, Ng MCH, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22–05): a single-arm, phase 1b–2 trial. Lancet Oncol. 2020;21:1066–76.
Catenacci DVT, Lim KH, Uronis HE, Kang Y-K, Ng MCH, Gold PJ, et al. Antitumor activity of margetuximab (M) plus pembrolizumab (P) in patients (pts) with advanced HER2+ (IHC3+) gastric carcinoma (GC). J Clin Oncol. 2019;37:65–65.
Combination Margetuximab, INCMGA00012, MGD013, and Chemotherapy Phase 2/3 Trial in HER2+ Gastric/GEJ Cancer (MAHOGANY)-Cerca con Google [Internet]. [cited 2020 Nov 7]. Available from: https://www.google.com/search?client=firefox-b-d&q=Combination+Margetuximab%2C+INCMGA00012%2C+MGD013%2C+and+Chemotherapy+Phase+2+%2F+3+Trial+in+HER2%2B+Gastric+%2F+GEJ+Cancer+%28MAHOGANY%29.
Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–108.
FDA approves new treatment option for patients with HER2-positive breast cancer who have progressed on available therapies. Case Med Res. 2019. http://www.fda.gov.
Iwata TN, Ishii C, Ishida S, Ogitani Y, Wada T, Agatsuma T. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther. 2018;17:1494–503.
Shi J, Li F, Yao X, Mou T, Xu Z, Han Z, et al. The HER4-YAP1 axis promotes trastuzumab resistance in HER2-positive gastric cancer by inducing epithelial and mesenchymal transition. Oncogene. 2018;37:3022–38.
Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, et al. Trastuzumab deruxtecan (DS-8201a)in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:827–36.
Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18:1512–22.
Shitara K, Bang Y-J, Iwasa S, Sugimoto N, Ryu M-H, Sakai D, et al. Trastuzumab Deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419–30.
Takegawa N, Tsurutani J, Kawakami H, Yonesaka K, Kato R, Haratani K, et al. [fam-] trastuzumab deruxtecan, antitumor activity is dependent on HER2 expression level rather than on HER2 amplification. Int J Cancer. 2019;145:3414–24.
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–46.
Mitani S, Kawakami H. Emerging targeted therapies for HER2 positive gastric cancer that can overcome Trastuzumab resistance. Cancers (Basel). 2020;12(2):400.
O’Donovan N, Byrne AT, O’Connor AE, McGee S, Gallagher WM, Crown J. Synergistic interaction between trastuzumab and EGFR/HER-2 tyrosine kinase inhibitors in HER-2 positive breast cancer cells. Invest New Drugs. 2011;29:752–9.
Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–11.
Minkovsky N, Berezov A. BIBW-2992, a dual receptor tyrosine kinase inhibitor for the treatment of solid tumors. Curr Opin Investig Drugs. 2008;9(12):1336–46.
Sanchez-Vega F, Hechtman JF, Castel P, Ku GY, Tuvy Y, Won H, et al. Egfr and MET amplifications determine response to HER2 inhibition in ERBB2-amplified esophagogastric cancer. Cancer Discov. 2019;9–2:199–209.
Kim TY, Han HS, Lee KW, Zang DY, Rha SY, Park YI, et al. A phase I/II study of poziotinib combined with paclitaxel and trastuzumab in patients with HER2-positive advanced gastric cancer. Gastric Cancer. 2019;22:1206–14.
Oh DY, Lee KW, Cho JY, Kang WK, Im SA, Kim JW, et al. Phase II trial of dacomitinib in patients with HER2-positive gastric cancer. Gastric Cancer. 2016;19:1095–103.
Coutzac C, Pernot S, Chaput N, Zaanan A. Immunotherapy in advanced gastric cancer, is it the future? Crit Rev Oncol Hematol. 2019;133(25):25–32.
Satoh T, Kang YK, Chao Y, Ryu MH, Kato K, Cheol Chung H, et al. Exploratory subgroup analysis of patients with prior trastuzumab use in the ATTRACTION-2 trial: a randomized phase III clinical trial investigating the efficacy and safety of nivolumab in patients with advanced gastric/gastroesophageal junction cancer. Gastric Cancer. 2020;23:143–53.
Kelly RJ, Chung K, Gu Y, Steele KE, Rebelatto MC, Robbins PB, et al. Phase Ib/II study to evaluate the safety and antitumor activity of durvalumab (MEDI4736) and tremelimumab as monotherapy or in combination, in patients with recurrent or metastatic gastric/gastroesophageal junction adenocarcinoma. J Immunother Cancer. 2015;3:p157.
Kelly RJ, Lee J, Bang Y-J, Almhanna K, Blum Murphy MA, Catenacci DVT, et al. Safety and efficacy of durvalumab in combination with tremelimumab, durvalumab monotherapy, and tremelimumab monotherapy in patients with advanced gastric cancer. J Clin Oncol. 2018;36:4031–4031.
Moehler M, Ryu MH, Dvorkin M, Lee KW, Coşkun H, Wong R, et al. Maintenance avelumab versus continuation of first-line chemotherapy in gastric cancer: JAVELIN Gastric 100 study design. Futur Oncol. 2019;15:567–77.
JapicCTI J. An Investigational Immuno-therapy Study to Assess the Safety, Tolerability and Effectiveness of Anti-LAG-3 With and Without Anti-PD-1 in the Treatment of Solid Tumors. 2018. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JapicCTI-183890.
Bang YJ, Yañez Ruiz E, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: Primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052–60.
Fornaro L, Vivaldi C, Parnofiello A, Ugolini C, Aprile G, De Maglio G, et al. Validated clinico-pathologic nomogram in the prediction of HER2 status in gastro-oesophageal cancer. Br J Cancer. 2019;120:522–6.
Lee HE, Park KU, Yoo SB, Nam SK, Park DJ, Kim HH, et al. Clinical significance of intratumoral HER2 heterogeneity in gastric cancer. Eur J Cancer. 2013;49:1448–57.
Palle J, Rochand A, Pernot S, Gallois C, Taïeb J, Zaanan A. Human Epidermal Growth Factor Receptor 2 (HER2) in advanced gastric cancer: current knowledge and future perspectives. Drugs. 2020;80(4):401–15.
Bozzetti C, Negri FV, Lagrasta CA, Crafa P, Bassano C, Tamagnini I, et al. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer. 2011;104:1372–6.
Park SR, Park YS, Ryu MH, Ryoo BY, Woo CG, Jung HY, et al. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur J Cancer. 2016;53:42–50.
Deguchi Y, Okabe H, Oshima N, Hisamori S, Minamiguchi S, Muto M, et al. PTEN loss is associated with a poor response to trastuzumab in HER2-overexpressing gastroesophageal adenocarcinoma. Gastric Cancer. 2017;20:416–27.
Kim C, Lee CK, Chon HJ, Kim JH, Park HS, Heo SJ, et al. PTEN loss and level of HER2 amplification is associated with trastuzumab resistance and prognosis in HER2-positive gastric cancer. Oncotarget. 2017;8:113494–501.
Huang LT, Ma JT, Zhang SL, Li XH, Sun L, Jing W, et al. Durable clinical response to Pyrotinib after resistance to prior anti-HER2 therapy for HER2-positive advanced gastric cancer: a case report. Front Oncol. 2019;9:1453.
Minuti G, Cappuzzo F, Duchnowska R, Jassem J, Fabi A, Obrien T, et al. Increased MET and HGF gene copy numbers are associated with trastuzumab failure in HER2-positive metastatic breast cancer. Br J Cancer. 2012;107:793–9.
Takahashi N, Furuta K, Taniguchi H, Sasaki Y, Shoji H, Honma Y, et al. Serum level of hepatocyte growth factor is a novel marker of predicting the outcome and resistance to the treatment with trastuzumab in HER2-positive patients with metastatic gastric cancer. Oncotarget. 2016;7:4925–38.
Kwak EL, Ahronian LG, Siravegna G, Mussolin B, Godfrey JT, Clark JW, et al. Molecular heterogeneity and receptor coamplification drive resistance to targeted therapy in MET-Amplified esophagogastric cancer. Cancer Discov. 2015;5:1271–81.
National Cancer Institute. The Cancer Genome Atlas Program [Internet]. Bethesda. 2020 [cited 2021 Jan 1]. Available from: https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga
Pietrantonio F, Fuca G, Morano F, Gloghini A, Corso S, Aprile G, et al. Biomarkers of primary resistance to trastuzumab in HER2-positive metastatic gastric cancer patients: the AMNESIA case-control study. Clin Cancer Res. 2018;24:1082–9.
Zang ZJ, Ong CK, Cutcutache I, Yu W, Zhang SL, Huang D, et al. Genetic and structural variation in the gastric cancer kinome revealed through targeted deep sequencing. Cancer Res. 2011;71:29–39.
Ucar DA, Kurenova E, Garrett TJ, Cance WG, Nyberg C, Cox A, et al. Disruption of the protein interaction between FAK and IGF-1R inhibits melanoma tumor growth. Cell Cycle. 2012;11:3250–9.
Arienti C, Zanoni M, Pignatta S, Del Rio A, Carloni S, Tebaldi M, et al. Preclinical evidence of multiple mechanisms underlying trastuzumab resistance in gastric cancer. Oncotarget. 2016;7:18424–39.
Gambardella V, Gimeno-Valiente F, Tarazona N, Ciarpaglini CM, Roda D, Fleitas T, et al. Nrf2 through RPs6 activation is related to anti-HER2 drug resistance in HER2-amplified gastric cancer. Clin Cancer Res. 2019;25:1639–49.
Piro G, Carbone C, Cataldo I, Di Nicolantonio F, Giacopuzzi S, Aprile G, et al. An FGFR3 autocrine loop sustains acquired resistance to trastuzumab in gastric cancer patients. Clin Cancer Res. 2016;22:6164–75.
Pietrantonio F, Caporale M, Morano F, Scartozzi M, Gloghini A, De Vita F, et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: implication for further clinical research. Int J Cancer. 2016;139:2859–64.
Mao L, Sun A-J, Wu J-Z, Tang J-H. Involvement of microRNAs in HER2 signaling and trastuzumab treatment. Tumor Biol. 2016;37(12):15437–46.
Sui M, Jiao A, Zhai H, Wang Y, Wang Y, Sun D, et al. Upregulation of miR-125b is associated with poor prognosis and trastuzumab resistance in HER2-positive gastric cancer. Exp Ther Med. 2017;14:657–63.
Kim HP, Han SW, Song SH, Jeong EG, Lee MY, Hwang D, et al. Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene. 2014;33:3334–41.
Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang Q, et al. Disrupting the interaction of BRD4 with Diacetylated twist suppresses Tumorigenesis in basal-like breast cancer. Cancer Cell. 2014;25:210–25.
Arigami T, Uenosono Y, Hirata M, Yanagita S, Ishigami S, Natsugoe S. B7–H3 expression in gastric cancer: A novel molecular blood marker for detecting circulating tumor cells. Cancer Sci. 2011;102(5):1019–24.
Tsujiura M, Ichikawa D, Konishi H, Komatsu S, ShiozakiOtsuji AE. Liquid biopsy of gastric cancer patients: circulating tumor cells and cell-free nucleic acids. World J Gastroenterol. 2014;20(12):3265–86.
Liu Y, Ling Y, Qi Q, Lan F, Zhu M, Zhang Y, et al. Prognostic value of circulating tumor cells in advanced gastric cancer patients receiving chemotherapy. Mol Clin Oncol. 2017;6:235–42.
Wang S, Zheng G, Cheng B, Chen F, Wang Z, Chen Y, et al. Circulating tumor cells (CTCs) detected by RT-PCR and its prognostic role in gastric cancer: a meta-analysis of published literature. PLoS One. 2014;9(6):e99259.
Riquet M, Rivera C, Gibault L, Pricopi C, Mordant P, Badia A, et al. Extension lymphatique du cancer du poumon: une anatomie enchaînée dans des zones. Rev Pneumol Clin. 2014;70:16–25.
Mavroudis D. Circulating cancer cells. Ann Oncol. 2010;21:vii95–100.
Tseng JY, Yang CY, Liang SC, Liu RS, Jiang JK, Lin CH. Dynamic changes in numbers and properties of circulating tumor cells and their potential applications. Cancers (Basel). 2014;6(4):2369–86.
Nevisi F, Yaghmaie M, Pashaiefar H, Alimoghaddam K, Iravani M, Javadi G, et al. Correlation of HER2, MDM2, c-MYC, c-MET, and TP53 copy number alterations in circulating tumor cells with tissue in gastric cancer patients: a pilot study. Iran Biomed J. 2020;24:47–53.
Mishima Y, Matsusaka S, Chin K, Mikuniya M, Minowa S, Takayama T, et al. Detection of HER2 amplification in circulating tumor cells of HER2-negative gastric cancer patients. Target Oncol. 2017;12:341–51.
Akca H, Demiray A, Yaren A, Bir F, Koseler A, Iwakawa R, et al. Utility of serum DNA and pyrosequencing for the detection of EGFR mutations in non-small cell lung cancer. Cancer Genet. 2013;206:73–80.
Neumann MHD, Bender S, Krahn T, Schlange T. ctDNA and CTCs in liquid biopsy-current status and where we need to progress. Comput Struct Biotechnol J. 2018;16:190–5.
Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24.
Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479–91.
Sumbal S, Javed A, Afroze B, Zulfiqar HF, Javed F, Noreen S, et al. Circulating tumor DNA in blood: future genomic biomarkers for cancer detection. Exp Hematol. 2018;65:17–28.
Kinugasa H, Nouso K, Tanaka T, Miyahara K, Morimoto Y, Dohi C, et al. Droplet digital PCR measurement of HER2 in patients with gastric cancer. Br J Cancer. 2015;112:1652–5.
Shoda K, Masuda K, Ichikawa D, Arita T, Miyakami Y, Watanabe M, et al. HER2 amplification detected in the circulating DNA of patients with gastric cancer: a retrospective pilot study. Gastric Cancer. 2015;18:698–710.
Maron SB, Chase LM, Lomnicki S, Kochanny S, Moore KL, Joshi SS, et al. Circulating tumor DNA sequencing analysis of gastroesophageal adenocarcinoma. Clin Cancer Res. 2019;25:7098–112.
Wang H, Li B, Liu Z, Gong J, Shao L, Ren J, et al. HER2 copy number of circulating tumour DNA functions as a biomarker to predict and monitor trastuzumab efficacy in advanced gastric cancer. Eur J Cancer. 2018;88:92–100.
Wang DS, Liu ZX, Lu YX, Bao H, Wu X, Zeng ZL, et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut. 2019;68(7):1152–61.
Peng Z, Liu Y, Li Y, Zhang X, Zhou J, Lu M, et al. Serum HER2 extracellular domain as a potential alternative for tissue HER2 status in metastatic gastric cancer patients. Biomark Med. 2014;8:663–70.
Witzel I, Loibl S, Von Minckwitz G, Eidtmann H, Fehm T, Khandan F, et al. Predictive value of HER2 serum levels in patients treated with lapatinib or trastuzumab-a translational project in the neoadjuvant GeparQuinto trial. Br J Cancer. 2012;107:956–60.
Lipton A, Leitzel K, Ali SM, Carney W, Platek G, Steplewski K, et al. Human epidermal growth factor receptor 2 (HER2) extracellular domain levels are associated with progression-free survival in patients with HER2-positive metastatic breast cancer receiving lapatinib monotherapy. Cancer. 2011;117:5013–20.
O’Donoghue JA, Lewis JS, Pandit-Taskar N, Fleming SE, Schöder H, Larson SM, et al. Pharmacokinetics, biodistribution, and radiation dosimetry for 89 Zr-trastuzumab in patients with esophagogastric cancer. J Nucl Med. 2018;59:161–6.
Bensch F, Brouwers AH, Lub-de Hooge MN, de Jong JR, van der Vegt B, Sleijfer S, et al. 89Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur J Nucl Med Mol Imaging. 2018;45:2300–6.
Janjigian YY, Viola-Villegas N, Holland JP, Divilov V, Carlin SD, Gomes-DaGama EM, et al. Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and89Zr-trastuzumab PET. J Nucl Med. 2013;54:936–43.
Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18(7):407–18.
Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172(373–386):e10.
Nanki K, Toshimitsu K, Takano A, Fujii M, Shimokawa M, Ohta Y, et al. Divergent routes toward Wnt and R-spondin Niche independency during human gastric carcinogenesis. Cell. 2018;174(856–869):e17.
Grün D, Van Oudenaarden A. Design and analysis of single-cell sequencing experiments. Cell. 2015;163(4):799–810.
Wang Q, Guldner IH, Golomb SM, Sun L, Harris JA, Lu X, et al. Single-cell profiling guided combinatorial immunotherapy for fast-evolving CDK4/6 inhibitor-resistant HER2-positive breast cancer. Nat Commun. 2019;10(1):3817.
Shah MA, XuBangHoffLiuHerráez-Baranda RHYJPMTLA, et al. HELOISE: phase IIIb randomized multicenter study comparing standard-of-care and higher-dose trastuzumab regimens combined with chemotherapy as first-line therapy in patients with human epidermal growth factor receptor 2–positive metastatic gastric or gast. J Clin Oncol. 2017;35(22):2558–67.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roviello, G., Aprile, G., D’Angelo, A. et al. Human epidermal growth factor receptor 2 (HER2) in advanced gastric cancer: where do we stand?. Gastric Cancer 24, 765–779 (2021). https://doi.org/10.1007/s10120-021-01182-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-021-01182-9