Abstract
Background
Gastric adenocarcinoma with enteroblastic differentiation (GAED) has been recognized as a variant of alpha-fetoprotein (AFP)-producing gastric carcinoma, although its clinicopathologic and immunohistochemical features have not been fully elucidated.
Methods
To elucidate the clinicopathologic and immunohistochemical features of GAED, we analyzed 29 cases of GAED, including ten early and 19 advanced lesions, and compared these cases with 100 cases of conventional gastric adenocarcinoma (CGA). Immunohistochemistry for AFP, glypican 3, SALL4, and p53 was performed, and the phenotypic expression of the tumors was evaluated by immunostaining with antibodies against MUC5AC, MUC6, MUC2, CD10, and caudal-type homeobox 2 (CDX2).
Results
Lymphatic and venous invasion was more frequent in GAED (76 and 72 %) than in CGA (41 and 31 %; P ≤ 0.001). Lymph node metastasis was more frequently observed in GAED (69 %) than in CGA (38 %; P = 0.005), as were synchronous or metachronous liver metastases (GAED, 31 %; CGA, 6 %; P ≤ 0.001). Immunohistochemically, all GAED were positive for at least one of three enteroblastic linage markers (AFP, glypican 3, and SALL4). Glypican 3 was the most sensitive marker (83 %) for GAED, followed by SALL4 (72 %) and AFP (45 %), whereas no CGA was positive. Furthermore, the rate of positive p53 staining was 59 % in GAED. Regarding the mucin phenotype, CD10 and CDX2 were diffusely or focally expressed in all GAED cases. Invasive areas with hepatoid or enteroblastic differentiation were negative for CD10 and CDX2.
Conclusions
Clinicopathologic features of GAED differ from those of CGA. GAED shows aggressive biological behavior, and is characteristically immunoreactive to AFP, glypican 3, or SALL4.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Gastric adenocarcinoma with enteroblastic differentiation (GAED), also known as clear cell gastric carcinoma, is a rare and not particularly well documented malignancy; previous reports have been either small series or a single case report [1, 2]. This type of tumor has been histologically characterized as having a primitive intestine-like structure, composed of cuboidal or columnar cells with clear cytoplasm [1, 3, 4]. GAED has also been noted to produce alpha-fetoprotein (AFP) in the serum and within the tumor [1, 2], and it is recognized as a variant of AFP-producing gastric carcinoma. However, the association between GAED and AFP production remains unclear as some GAED cases may be AFP negative [2, 5].
Since the first case of AFP-producing gastric cancer with liver metastasis was reported in 1970 [6], many cases of this tumor type have been reported. In previous reports, AFP-producing gastric cancer was associated with a poor prognosis, and advanced-stage disease usually presented with liver metastases [7–10]. Histologically, typical AFP-producing gastric cancer shows a hepatoid pattern composed of neoplastic cells with abundant eosinophilic cytoplasm in solid nests [11], or a clear cell tubular pattern resembling fetal gut epithelium [1, 3, 4]. Diagnosis of AFP-producing gastric cancer is based on positive immunohistochemical staining of AFP; however, some hepatoid or enteroblastic tumors are negative for AFP expression.
Recent studies have demonstrated that glypican 3 and SALL4 are also oncofetal proteins indicative of hepatocyte differentiation [4, 12–16]. Glypican 3, a cell-surface heparan sulfate proteoglycan, is present in fetal liver and hepatocellular carcinoma or hepatoblastoma [4, 12–14]. Immunohistochemically, glypican 3-positive areas nearly always overlap with AFP-positive areas in AFP-producing gastric cancer [13]. SALL4 is a member of the SALL gene family and acts as a zinc finger transcription factor. SALL4 has an essential role in maintaining the self-renewal and pluripotency of embryonic stem cells [15, 16]. Accordingly, glypican 3 and SALL4 have been suggested to be sensitive markers for AFP-producing gastric cancer and related tumors.
Thus, the aim of this study was to elucidate the clinicopathologic and immunohistochemical features of GAED in association with the immunostaining of AFP, glypican 3, SALL4, and p53.
Materials and methods
Case selection
We studied 29 patients with GAED who underwent endoscopic or surgical resection at Juntendo University Hospital, Tokyo, Japan, between January 2009 and March 2014. These included 10 early and 19 advanced lesions. The diagnosis of GAED was based on the criteria described by Matsunou et al. [1]: (1) columnar carcinoma cells grow primarily in tubulopapillary and glandular patterns; (2) carcinoma cells have clear cytoplasm and an oval nucleus situated on the basal side; (3) abundant glycogen granules, but no mucin, are contained in the clear cytoplasm.
Sex, age, tumor location, tumor size, macroscopic appearance, depth of invasion (pT stage), lymphatic or venous invasion, lymph node metastasis, hepatic metastasis, and outcome were evaluated in all patients. The pT stage was classified according to the seventh edition of the American Joint Committee on Cancer/Union for International Cancer Control staging system [17]. We classified pT1 tumors irrespective of lymph node or liver metastasis as early lesions, and we defined the remaining tumors as advanced lesions.
Immunohistochemistry
Serial tissue sections (4 mm thick) prepared from formalin-fixed and paraffin-embedded tissues were subjected to immunohistochemistry. For immunohistochemical examination, staining was performed using a Dako EnVision kit with antibodies against AFP (rabbit polyclonal, 1:1000; Dako, Glostrup, Denmark), glypican 3 (clone 1G12, 1:200; BioMosaics, Burlington, VT, USA) SALL4 (clone 6E3, 1:100; Abnova, Taipei, Taiwan), and p53 (clone DO-7, 1:100; Dako, Glostrup, Denmark). The phenotypic expression of the tumors was evaluated by immunostaining with antibodies against MUC5AC (NCL-MUC-5AC, 1:100; Novocastra, Newcastle-upon-Tyne, UK), MUC6 (NCL-MUC-6, 1:100; Novocastra, Newcastle upon Tyne, UK), MUC2 (NCL-MUC-2, 1:100; Novocastra, Newcastle upon Tyne, UK), CD10 (NCL-CD10-270, 1:100; Novocastra, Newcastle upon Tyne, UK), and caudal-type homeobox 2 (CDX2; CDX2-88, 1:100; BioGenex, Fremont, CA, USA). Appropriate positive and negative controls were used for each antibody.
AFP, glypican 3, or SALL4 staining was assessed according to a previous description [15]. Cytoplasmic staining for AFP, and membrane and cytoplasmic staining for glypican 3 were evaluated. Only nuclear staining was considered positive for SALL4. The scoring system was as follows: score 0, less than 1 % of tumor cells positive; score 1, 1–25 % of tumor cells positive; score 2, 26–50 % of tumor cells positive; score 3, 51–75 % of tumor cells positive; and score 4, more than 75 % of tumor cells positive. The final results were reported as negative (score 0) or positive (score 1, 2, 3, or 4).
The phenotypes were classified into four categories according to the combination of the expression of CD10, MUC2, MUC5AC, MUC6, and CDX2 [18]. Specimens positive for MUC5AC or MUC6 were defined as gastric type, those positive for MUC2, CD10, or CDX2 were defined as intestinal type, and those with both phenotypes were considered to be gastrointestinal type. In addition, specimens with no CD10, MUC2, MUC5AC, or MUC6 expression were considered to be unclassified type. Expression of p53 was recorded as positive if distinct and strong nuclear staining was observed in more than10 % of tumor cells [19].
The histology and immunohistochemical staining results were evaluated by two observers (T.M. and T.Y.). When discrepancies arose, the cases were reviewed using a multiheaded microscope to achieve a consensus.
Comparisons with conventional gastric adenocarcinoma
We randomly selected 100 samples of conventional gastric adenocarcinoma (CGA) from our files of endoscopically or surgically resected specimens at our hospital between 2009 and 2014. These lesions were histologically papillary adenocarcinomas, well to moderately differentiated tubular adenocarcinomas, and poorly differentiated adenocarcinomas. We compared 29 GAED with 100 CGA in regard to clinicopathologic findings such as sex, age, tumor location, tumor size, macroscopic appearance, pT stage, lymphatic or venous invasion, lymph node metastasis, and hepatic metastasis. Furthermore, we randomly selected 20 lesions from those 100 CGA, and performed immunohistochemical staining using antibodies against AFP, glypican 3, and SALL4 as described earlier.
Statistical analysis
All statistical analyses were performed using Stat-View for Windows version 5.0 (SAS Institute, Cary, NC, USA). Continuous data were compared with the Mann–Whitney U test. Categorical analysis of variables was performed using either the chi squared test (with Yates’s correction) or Fisher’s exact test, as appropriate. A P value less than 0.05 was considered statistically significant.
Results
Clinicopathologic findings
The clinicopathologic findings of the 29 GAED patients and the 100 CGA patients included in this study are summarized in Table 1, and detailed clinicopathologic findings of 29 GAED lesions are listed in Table 2. There were 23 male and six female GAED patients, with ages ranging from 59 to 85 years (mean 73 years). Six GAED were located in the upper area of the stomach, nine were in the middle area, and 14 were in the lower area. The mean tumor sizes of GAED were 19 mm for early lesions and 49 mm for advanced lesions (P < 0.001). Macroscopically, superficially depressed-type lesions were frequently observed among early GAED cases, and types 2 and 3 were frequently observed among advanced GAED cases, similarly to CGA cases. With regard to the depth of invasion, in the 29 GAED patients and the 100 CGA patients, pT1 was observed in ten and 33 patients, respectively, pT2 was observed in four and 16 patients, respectively, pT3 was observed in 12 and 37 patients, respectively, and pT4 was observed in three and 14 patients, respectively. Neither of these comparisons was considered statistically significant.
Lymphatic invasion was more frequently observed in GAED (22 of 29 cases, 76 %) than in CGA (41 of 100 cases, 41 %; P = 0.001), as was venous invasion (GAED, 72 %; CGA, 31 %; P ≤ 0.001). Lymphatic and/or venous invasion was present in 26 GAED (90 %), including eight early lesions and 18 advanced lesions. Lymph node metastasis was more frequently observed in GAED (20 of 29 cases, 69 %), including four early cancer patients and 16 advanced cancer patients, than in CGA (38 of 100 cases, 38 %; P = 0.005. Synchronous or metachronous liver metastases were more frequently found in GAED (nine of 29 cases, 31 %) than in CGA (six of 100 cases, 6 %; P ≤ 0.001).
Histopathologic findings
The histopathologic findings of the 29 GAED specimens are summarized in Table 2. All 29 cases showed adenocarcinoma composed of cuboidal or columnar cells with clear cytoplasm, resembling the primitive gut (Fig. 1a). In cases 6, 11, and 22, part of the invasive area exhibited a hepatoid pattern (Fig. 1b); in case 27, the tumor partly exhibited a yolk sac tumor pattern, representing reticular or papillary structures composed of cuboidal or columnar cells (Fig. 1c). Enteroblastic adenocarcinoma was combined with conventional well-differentiated or moderately-differentiated tubular adenocarcinoma in the upper part of all tumors (Fig. 1d).
Histologic features of gastric adenocarcinoma with enteroblastic differentiation. a A major portion of the lesion in case 16 was composed of cuboidal or columnar cells with clear cytoplasm resembling the primitive gut. b A minor portion of the lesion in case 11 exhibited hepatoid adenocarcinoma. c In case 27, the tumor partly exhibited a yolk sac tumor pattern, representing reticular or papillary structures composed of cuboidal or columnar cells. d Enteroblastic adenocarcinoma was combined with conventional well-differentiated or moderately-differentiated tubular adenocarcinoma in the upper part of the tumor in case 9
Outcomes
The duration of follow-up ranged from 2 to 39 months (mean, 18.4 months; Table 2). Patient 10 was lost to follow-up 2 months after endoscopic therapy. Complete follow-up clinical data were obtained from the remaining 28 GAED patients. Fifteen patients, including two early cancer patients and 13 advanced cancer patients, died as a result of their disease at an average of 14.6 months after surgery. Moreover, all nine patients with liver metastasis died within 3 years of surgery.
Immunohistochemical findings
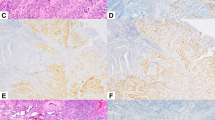
The results of the immunohistochemical analyses are summarized in Table 3. All GAED were positive for at least one of three enteroblastic linage markers (AFP, glypican 3, and SALL4; Fig. 2). Glypican 3 was the most sensitive marker (positivity, 83 %), followed by SALL4 (72 %) and AFP (45 %). Diffuse marker positivity (score 2, 3, or 4) was more frequently identified in advanced lesions (AFP, 26 %; glypican 3, 47 %; SALL4, 58 %) than in early lesions (AFP, 10 %; glypican 3, 20 %; SALL4, 30 %). By contrast, there was no expression of AFP, glypican 3, and SALL4 in 20 cases of CGA. In addition, the rate of positive p53 staining was 59 % in all GAED lesions. With regard to phenotypic expression, 15 GAED cases (52 %) exhibited the pure intestinal phenotype, whereas the remaining 14 cases (48 %) exhibited the gastrointestinal phenotype. CD10 and CDX2 were diffusely or focally expressed in all GAED cases (Fig. 2d).
Immunohistochemical staining of gastric adenocarcinoma with enteroblastic differentiation. Positive findings for alpha-fetoprotein in case 11 (a), for glypican 3 in case 23 (b), for SALL4 in case 11 (c), and for caudal-type homeobox 2 in case 25 (d). In case 11, the tumor exhibited an enteroblastic structure (e, left) accompanied by a hepatoid structure (e, right). f The immunostaining of CD10 in the same area as that shown in e. CD10 was expressed preferentially in the enteroblastic area (f, left), but not in the hepatoid area (f, right)
Association of marker expression and tumor invasion, metastases, or outcomes
In GAED cases, we further analyzed the associations of mucin phenotype and expression of AFP, glypican 3, SALL4, and p53 with venous invasion, lymphatic invasion, lymph node metastasis, hepatic metastasis, and outcome. There were no significant associations, although glypican 3 and SALL4 expression tended to be associated with lymphatic invasion (glypican 3, P = 0.069; SALL4, P = 0.074).
Discussion
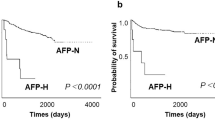
In this study, patients with GAED were predominantly male (79 %), and GAED lesions were frequently located in the middle third and lower third of the stomach (79 %), similarly to CGA [20–22]. The mean age of patients at diagnosis tended to be older than that of patients with CGA [20–22]. Lymphatic and vascular involvements are common among AFP-producing gastric carcinomas [10]. The prevalence of lymphatic involvement and that of venous involvement in GAED (76 and 72 %, respectively) were remarkably higher than those in CGA (10–56 %) [21, 23]. In this study, most GAED patients had lymphatic and/or vascular invasion (90 %). Lymph node metastasis was detected in 40 % of early lesions and 84 % of advanced lesions, and these rates are also higher than those associated with CGA (26–45 %) [20, 21]. Furthermore, 31 % of GAED patients had synchronous or metachronous liver metastasis. Our findings were in accordance with a review of 270 patients with AFP-producing gastric carcinoma, which reported an 83 % incidence of lymph node metastasis and a 33 % incidence of liver metastasis [10]. AFP-producing gastric cancers are typically associated with a poor prognosis and high rates of liver metastasis [9]. Hence, our data suggest that GAED, as well as AFP-producing gastric cancer, is associated with a poor prognosis.
With the exception of hepatocellular carcinoma or yolk sac tumors, gastric cancer is one of the commonest AFP-producing tumors [4, 6–10, 24–27]. Gastric hepatoid adenocarcinoma or GAED is one of the representative histologic types of AFP-producing gastric cancer. AFP production is generally considered the result of retrodifferentiation of tumor cells into fetal cells capable of producing AFP [1, 28]; therefore, this suggests that gastric hepatoid adenocarcinoma and GAED exhibit retrodifferentiation. However, some hepatoid tumors are negative for AFP expression, and do not have the ability to produce AFP. Similarly, the rate of negative AFP staining was 55 % in GAED, suggesting that some GAED do not have the ability to produce AFP. The histogenesis of AFP-producing gastric carcinoma still remains unclear. AFP-producing gastric carcinoma is thought to develop from conventional tubular adenocarcinoma, subsequently progressing to GAED with hepatoid differentiation and AFP production [4]. Accordingly, we also found that GAED lesions were mixed with conventional well-differentiated or moderately-differentiated tubular adenocarcinoma in all cases.
Recent studies have demonstrated that SALL4 is specifically expressed not only in primitive germ cell tumors but also in gastric carcinoma with fetal gut differentiation [15, 16]. In our study, expression of SALL4 was positive in 20 cases (72 %), which was higher than AFP positivity (45 %), indicating that SALL4 was a more sensitive marker of fetal gut differentiation than AFP. Ushiku et al. [15] stated that SALL4 is a sensitive marker for AFP-producing gastric carcinoma and is especially useful to diagnose hepatoid gastric carcinoma. The previous report and our findings suggest that SALL4 is useful to diagnose not only hepatoid gastric carcinoma but also a series of variants of gastric carcinoma with retrodifferentiation such as GAED. Glypican 3 is also a sensitive marker of hepatoid components of AFP-producing gastric carcinoma [4, 12–14]. In the present study, glypican 3 expression was observed in 24 cases (83 %), and was thereby the most sensitive marker for GAED. The Zhx2 gene, the mouse orthologue of human ZHX2, acts as a repressor of both AFP and glypican 3 expression in the adult mouse liver [29, 30], and it is often silenced in hepatocellular carcinoma in association with Zhx2 promoter hypermethylation. In addition, ZHX2 mRNA levels were decreased in hepatocellular carcinoma cases with high serum AFP levels [31]. It is of further interest to investigate the relationship between ZHX2 repression and glypican 3 or AFP expression in GAED.
Protein p53 plays a crucial role in cell apoptosis. Wild-type p53 induces growth arrest at the G1/S phase of the cell cycle in response to DNA damage, thus inhibiting the proliferation of cells [32]. Mutated p53 loses this inhibitive function, thus allowing cells with damaged DNA to proliferate. In this study, we found that p53 expression did not correlate with clinicopathologic parameters [33]. It is controversial whether p53 expression predicts prognosis; however, some studies have reported that p53 has no association with prognosis in conventional gastric cancer or AFP-producing gastric cancer patients [34–36], a finding similar to that in our study.
With regard to mucin phenotype, most AFP-producing gastric carcinomas are classified as the intestinal type [4, 37], in accordance with our study. Invasive areas with hepatoid or enteroblastic differentiation were negative for CD10 and CDX2 expression, suggesting the retrodifferentiation of tumor cells (Fig. 2e, f).
In conclusion, GAED, resembling the primitive gut, showed aggressive behavior such as lymphatic and venous invasion, lymph node metastasis, and liver metastasis, and its clinicopathologic features were similar to those of AFP-producing gastric carcinoma but were not similar to those of CGA. In addition, GAED was immunoreactive to AFP, glypican 3, or SALL4. These findings indicate that some GAED have the ability to produce AFP and overlap with AFP-producing gastric carcinoma.
References
Matsunou H, Konishi F, Jalal RE, Yamamichi N, Mukawa A. Alpha-fetoprotein-producing gastric carcinoma with enteroblastic differentiation. Cancer. 1994;73:534–40.
Govender D, Ramdial PK, Clarke B, Chetty R. Clear cell (glycogen-rich) gastric adenocarcinoma. Ann Diagn Pathol. 2004;8:69–73.
Kodama T, Kameya T, Hirota T, Shimosato Y, Ohkura H, Mukojima T, et al. Production of alpha-fetoprotein, normal serum proteins, and human chorionic gonadotropin in stomach cancer: histologic and immunohistochemical analyses of 35 cases. Cancer. 1981;48:1647–55.
Kinjo T, Taniguchi H, Kushima R, Sekine S, Oda I, Saka M, et al. Histologic and immunohistochemical analyses of α-fetoprotein-producing cancer of the stomach. Am J Surg Pathol. 2012;36:56–65.
Ghotli ZA, Serra S, Chetty R. Clear cell (glycogen rich) gastric adenocarcinoma: a distinct tubulo-papillary variant with a predilection for the cardia/gastro-oesophageal region. Pathology. 2007;39:466–9.
Bourreille J, Metayer P, Sauger F, Matray F, Fondimare A. Existence of alpha feto protein during gastric-origin secondary cancer of the liver. Presse Med. 1970;78:1277–8.
Chang YC, Nagasue N, Kohno H, Taniura H, Uchida M, Yamanoi A, et al. Clinicopathologic features and long-term results of alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol. 1990;85:1480–5.
Chang YC, Nagasue N, Abe S, Taniura H, Kumar DD, Nakamura T. Comparison between the clinicopathologic features of AFP-positive and AFP-negative gastric cancers. Am J Gastroenterol. 1992;87:321–5.
Kono K, Amemiya H, Sekikawa T, Iizuka H, Takahashi A, Fujii H, et al. Clinicopathologic features of gastric cancers producing alpha-fetoprotein. Dig Surg. 2002;19:359–65.
Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003;65:95–101.
Ishikura H, Kirimoto K, Shamoto M, Miyamoto Y, Yamagiwa H, Itoh T, et al. Hepatoid adenocarcinomas of the stomach. An analysis of seven cases. Cancer. 1986;58:119–26.
Yamauchi N, Watanabe A, Hishinuma M, Ohashi K, Midorikawa Y, Morishita Y, et al. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005;18:1591–8.
Hishinuma M, Ohashi KI, Yamauchi N, Kashima T, Uozaki H, Ota S, et al. Hepatocellular oncofetal protein, glypican 3 is a sensitive marker for α-fetoprotein-producing gastric carcinoma. Histopathology. 2006;49:479–86.
Ushiku T, Uozaki H, Shinozaki A, Ota S, Matsuzaka K, Nomura S, et al. Glypican 3-expressing gastric carcinoma: distinct subgroup unifying hepatoid, clear-cell, and α-fetoprotein-producing gastric carcinomas. Cancer Sci. 2009;100:626–32.
Ushiku T, Shinozaki A, Shibahara J, Iwasaki Y, Tateishi Y, Funata N, et al. SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in distinguishing hepatoid gastric carcinoma from hepatocellular carcinoma. Am J Surg Pathol. 2010;34:533–40.
Ikeda H, Sato Y, Yoneda N, Harada K, Sasaki M, Kitamura S, et al. α-Fetoprotein-producing gastric carcinoma and combined hepatocellular and cholangiocarcinoma show similar morphology but different histogenesis with respect to SALL4 expression. Hum Pathol. 2012;43:1955–63.
Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–9.
Kabashima A, Yao T, Sugimachi K, Tsuneyoshi M. Gastric or intestinal phenotypic expression in the carcinomas and background mucosa of multiple early gastric carcinomas. Histopathology. 2000;37:513–22.
Oh SY, Kwon HC, Kim SH, Jang JS, Kim MC, Kim KH, et al. Clinicopathologic significance of HIF-1α, p53, and VEGF expression and preoperative serum VEGF level in gastric cancer. BMC Cancer. 2008;8:123. doi:10.1186/1471-2407-8-123.
Maruyama K, Gunvén P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg. 1989;1989(210):596–602.
Maehara Y, Emi Y, Baba H, Adachi Y, Akazawa K, Ichiyoshi Y, et al. Recurrences and related characteristics of gastric cancer. Br J Cancer. 1996;74:975–9.
Selcukbiricik F, Buyukunal E, Tural D, Ozguroglu M, Demirelli F, Serdengecti S. Clinicopathological features and outcomes of patients with gastric cancer: a single-center experience. World J Gastroenterol. 2013;19:2154–61.
Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301–16.
Saito S, Hatano T, Hayakawa M, Koyama Y, Ohsawa A, Iwamasa T. Studies on alpha-fetoprotein produced by renal cell carcinoma. Cancer. 1989;63:544–9.
Hocking GR, Shembrey M, Hay D, Ostör AG. Alpha-fetoprotein-producing adenocarcinoma of the sigmoid colon with possible hepatoid differentiation. Pathology. 1995;27:277–9.
Yamagata T, Yamagata Y, Nakanishi M, Matsunaga K, Minakata Y, Ichinose M. A case of primary lung cancer producing alpha-fetoprotein. Can Respir J. 2004;11:504–6.
Isonishi S, Ogura A, Kiyokawa T, Suzuki M, Kunito S, Hirama M, et al. Alpha-fetoprotein (AFP)-producing ovarian tumor in an elderly woman. Int J Clin Oncol. 2009;14:70–3.
Eom BW, Jung SY, Yoon H, Kook MC, Ryu KW, Lee JH, et al. Gastric choriocarcinoma admixed with an α-fetoprotein-producing adenocarcinoma and separated adenocarcinoma. World J Gastroenterol. 2009;15:5106–8.
Perincheri S, Dingle RW, Peterson ML, Spear BT. Hereditary persistence of α-fetoprotein and H19 expression in liver of BALB/cJ mice is due to a retrovirus insertion in the Zhx2 gene. Proc Natl Acad Sci U S A. 2005;102:396–401.
Morford LA, Davis C, Jin L, Dobierzewska A, Peterson ML, Spear BT. The oncofetal gene glypican 3 is regulated in the postnatal liver by zinc fingers and homeoboxes 2 and in the regenerating liver by alpha-fetoprotein regulator 2. Hepatology. 2007;46:1541–7.
Lv Z, Zhang M, Bi J, Xu F, Hu S, Wen J. Promoter hypermethylation of a novel gene, ZHX2, in hepatocellular carcinoma. Am J Clin Pathol. 2006;125:740–6.
Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Nad Acad Sci U S A. 1992;89:7491–5.
Livingstone LR, White A, Sprouse J, Livanos E, Jacks T, Tlsty TD. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992;70:923–35.
Motojima K, Furui J, Kohara N, Ito T, Kanematsu T. Expression of p53 protein in gastric carcinomas is not independently prognostic. Surgery. 1994;116:890–5.
Gabbert HE, Müller W, Schneiders A, Meier S, Hommel G. The relationship of p53 expression to the prognosis of 418 patients with gastric carcinoma. Cancer. 1995;76:720–6.
Liu X, Yu H, Cai H, Wang Y. Expression of CD24, p21, p53, and c-myc in alpha-fetoprotein-producing gastric cancer: correlation with clinicopathologic characteristics and survival. J Surg Oncol. 2014;109:859–64.
Akiyama S, Tamura G, Endoh Y, Fukushima N, Ichihara Y, Aizawa K, et al. Histogenesis of hepatoid adenocarcinoma of the stomach: molecular evidence of identical origin with coexistent tubular adenocarcinoma. Int J Cancer. 2003;106:510–5.
Acknowledgments
We thank Noriko Sasahara and Isao Kurahayashi (Department of Human Pathology, Juntendo University School of Medicine) for their assistance with the histologic analysis.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murakami, T., Yao, T., Mitomi, H. et al. Clinicopathologic and immunohistochemical characteristics of gastric adenocarcinoma with enteroblastic differentiation: a study of 29 cases. Gastric Cancer 19, 498–507 (2016). https://doi.org/10.1007/s10120-015-0497-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-015-0497-9