Abstract
Background
We aimed to clarify the relationship between the maximum tolerated dose and plasma concentration of paclitaxel in Japanese patients with gastric cancer on a weekly paclitaxel administration regimen.
Methods
Thirty-three patients with advanced or recurrent gastric cancer were treated with escalating doses of paclitaxel, administered weekly, along with a fixed dose of 5-fluorouracil or cisplatin.
Results
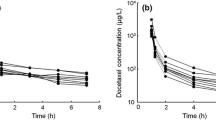
The plasma concentration of paclitaxel remained above 8.5 ng/ml for 24 h after administration. The mean area under the curve increased significantly with escalating dosage levels (R = 0.63; P 0.001). At level 4, patients showing dose-limiting toxicity had a significantly higher plasma paclitaxel concentration than patients without it.
Conclusion
The weekly administration of paclitaxel, for which a single dose is about one-third of the dose for a tri-weekly treatment regimen, is clinically feasible and appropriate in terms of toxicity and the maintenance of an effective plasma concentration.
Article PDF
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
References
EK Rowinsky RC Donehower (1995) ArticleTitlePaclitaxel (Taxol) N Engl J Med 332 1004–14 Occurrence Handle7885406 Occurrence Handle10.1056/NEJM199504133321507 Occurrence Handle1:STN:280:ByqC1M7mtFQ%3D
Y-F Chang LL Li C-W Wu W-Y Liu F-K P'eng C-W Chi (1996) ArticleTitlePaclitaxel-induced apoptosis in human gastric carcinoma cell lines Cancer 77 14–8 Occurrence Handle8630921 Occurrence Handle10.1002/(SICI)1097-0142(19960101)77:1<14::AID-CNCR4>3.0.CO;2-N Occurrence Handle1:CAS:528:DyaK28XlsV2guw%3D%3D
HY Kim SW Shin BS Kim JH Kim JG Kim YJ Mok et al. (1999) ArticleTitlePaclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma Cancer 85 295–301 Occurrence Handle10023695 Occurrence Handle10.1002/(SICI)1097-0142(19990115)85:2<295::AID-CNCR5>3.0.CO;2-H Occurrence Handle1:CAS:528:DyaK1MXhtFGgt7w%3D
AM Murad A Petroianu RC Guimaraes BC Aragao LOM Cabral AO Scalabrini-Neto (1999) ArticleTitlePhase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer. A novel, safe, and effective regimen Am J Clin Oncol 22 580–6 Occurrence Handle10597742 Occurrence Handle10.1097/00000421-199912000-00008 Occurrence Handle1:STN:280:DC%2BD3c%2FmsVKisA%3D%3D
JJ Lokich H Sonneborn NR Anderson MM Bern FV Coco E Dow P Oliynyk (1999) ArticleTitleCombined paclitaxel, cisplatin, and etoposide for patients with previously untreated esophageal and gastroesophageal carcinomas Cancer 85 2347–51 Occurrence Handle10357404 Occurrence Handle10.1002/(SICI)1097-0142(19990601)85:11<2347::AID-CNCR8>3.0.CO;2-8 Occurrence Handle1:CAS:528:DyaK1MXjvFyjtL0%3D
C Kollmannsberger D Quietzsch C Haag T Lingenfelser M Schroeder JT Hartmann et al. (2000) ArticleTitleA phase II study of paclitaxel, weekly, 24-hour continuous infusion 5-fluorouracil, folinic acid and cisplatin in patients with advanced gastric cancer Br J Cancer 83 458–62 Occurrence Handle10945491 Occurrence Handle10.1054/bjoc.2000.1295 Occurrence Handle1:CAS:528:DC%2BD3cXmtFOmt70%3D
AA Garcia CG Leichman HJ Lenz J Baranda R Lujan Y Casagrande L Leichman (2001) ArticleTitlePhase II trial of outpatient schedule of paclitaxel in patients with previously untreated metastatic, measurable adenocarcinoma of the stomach Jpn J Clin Oncol 31 275–8 Occurrence Handle11463806 Occurrence Handle10.1093/jjco/hye060 Occurrence Handle1:STN:280:DC%2BD3MvhsVGhtA%3D%3D
Y Yamada K Shirao A Ohtsu N Boku I Hyodo Y Miyata T Taguchi (2001) ArticleTitlePhase II trial of paclitaxel by 3-hour infusion for advanced gastric cancer with short premedication for prophylaxis against paclitaxel-associated hypersensitivity reactions Ann Oncol 12 1133–7 Occurrence Handle11583196 Occurrence Handle10.1023/A:1011680507956 Occurrence Handle1:STN:280:DC%2BD3MrjsFehtQ%3D%3D
H Matsuoka K Yano Y Saito H Tomoda (1995) ArticleTitleCytotoxicity of paclitaxel in comparison with other anticancer agents against neoplastic cells obtained from clinical gastrointestinal carcinoma tissue Anticancer Res 15 2001–6 Occurrence Handle8572592 Occurrence Handle1:CAS:528:DyaK28XhsFWhtrc%3D
D Fennelly C Aghajanian F Shapiro C O'Flaherty M McKenzie C O'Connor et al. (1997) ArticleTitlePhase I and pharmacologic study of paclitaxel administered weekly in patients with relapsed ovarian cancer J Clin Oncol 15 187–92 Occurrence Handle8996141 Occurrence Handle1:CAS:528:DyaK2sXnsV2ntA%3D%3D
U Klaassen H Wilke D Stumberg W Eberhardt M Korn S Seeber (1996) ArticleTitlePhase I study with a weekly 1-h infusion of paclitaxel in heavily pretreated patients with metastatic breast and ovarian cancer Eur J Cancer 32A 547–9 Occurrence Handle8814705 Occurrence Handle10.1016/0959-8049(95)00641-9 Occurrence Handle1:CAS:528:DyaK28XisFyitbs%3D
Y Segawa K Watanabe S Hiraki K Tominaga I Hayashi M Harada et al. (2000) ArticleTitlePhase I study of docetaxel and cisplatin for patients with previously untreated metastatic non-small-cell lung cancer: a Japanese cooperative study Int J Clin Oncol 5 308–15 Occurrence Handle10.1007/PL00012055
Y Kano M Akutsu S Tsunoda J Matsui K Suzuki T Ikeda et al. (1996) ArticleTitleSchedule-dependent interaction between paclitaxel and 5-fluorouracil in human carcinoma cell lines in vitro Br J Cancer 74 704–10 Occurrence Handle8795571 Occurrence Handle1:CAS:528:DyaK28XmtF2mur0%3D
R Shimon EL Karn (1990) ArticleTitleSelecting drug combinations based on total equivalent dose (dose intensity) J Natl Cancer Inst 82 1469–76 Occurrence Handle10.1093/jnci/82.18.1469
P Pronzato G Bertelli A Vigani F Vaira (1996) ArticleTitleA feasibility study of accelerated polychemotherapy with cisplatin, epidoxorubicin, and cyclophosphamide (PEC) in advanced ovarian cancer Br J Cancer 73 1425–7 Occurrence Handle8645591 Occurrence Handle1:CAS:528:DyaK28XksF2ktLw%3D
InstitutionalAuthorNameNational Cancer Institute (1993) NCI common toxicity criteria, version 1 Investigators handbook: a manual of participants in clinical trials of investigational agents, sponsored by the Division of Cancer Treatment, National Cancer Institute U.S. Department of Health and Human Services, Public Health Service, National Institute of Health Bethesda, MD
PH Wiernik EL Schwartz A Einzig JJ Strauman RB Lipton JP Cutcher (1987) ArticleTitlePhase I trial of taxol given as a 24-hour infusion every 21 days: responses observed in metastatic melanoma J Clin Oncol 5 1232–9 Occurrence Handle2887641 Occurrence Handle1:STN:280:BiiA3M%2Fls1I%3D
U Vanhoefer P Rougier H Wilke MP Ducreux AJ Lacave E Van Cutsem et al. (2000) ArticleTitleFinal result of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group J Clin Oncol 18 2648–57 Occurrence Handle10894863 Occurrence Handle1:CAS:528:DC%2BD3cXls12iurk%3D
A Ohtsu Y Shimada S Yoshida H Saito S Seki K Morise M Kurihara (1994) ArticleTitlePhase II study of protracted infusional 5-fluorouracil combined with cisplatinum for advanced gastric cancer: report from the Japan Clinical Oncology Group (JCOG) Eur J Cancer 30A 2091–3 Occurrence Handle7857709 Occurrence Handle10.1016/0959-8049(94)00350-E Occurrence Handle1:STN:280:ByqC287hsVI%3D
Y Shimada K Shirao A Ohtsu I Hyodo H Saito N Yamamichi et al. (1999) ArticleTitlePhase III study of UFT + MMC versus 5-FU + CDDP versus 5-FU alone in patients with advanced gastric cancer. JCOG study 9205 (abstract) Proc ASCO 18 272
K Yamaguchi M Tada N Horikoshi T Otani H Takiuchi S Saitoh et al. (2002) ArticleTitlePhase II study of paclitaxel with 3-h infusion in patients with advanced gastric cancer Gastric Cancer 5 90–5 Occurrence Handle12111584 Occurrence Handle10.1007/s101200200015 Occurrence Handle1:CAS:528:DC%2BD38XmslGlsbg%3D
P Rosenberg H Anderson K Boman M Ridderheim B Sorbe U Puistola G Horvath (1999) ArticleTitleA randomized multicenter study of single agent paclitaxel given weekly versus every 3 weeks to patients with ovarian cancer previously treated with platinum therapy (abstract) Proc ASCO 18 368a
L Norton (2001) ArticleTitleTheoretical concepts and the emerging role of taxanes in adjuvant therapy Oncologist 6 IssueIDSuppl 3 30–5 Occurrence Handle11346683 Occurrence Handle10.1634/theoncologist.6-suppl_3-30 Occurrence Handle1:CAS:528:DC%2BD3MXktValurs%3D
MJ Glantz H Choy W Akerley CM Kearns MJ Egorin CH Rhodes BF Cole (1996) ArticleTitleWeekly paclitaxel with and without concurrent radiation therapy: toxicity, pharmacokinetics, and response Semin Oncol 23 IssueID6 Suppl 16 128–35 Occurrence Handle9007140 Occurrence Handle1:CAS:528:DyaK2sXhtVeiu7c%3D
MM Oken RH Creech DC Tormey J Horton TE Davis ET McFadden et al. (1982) ArticleTitleToxicity and response criteria of the Eastern Cooperative Oncology Group Am J Clin Oncol 6 649–55
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kobayashi, M., Oba, K., Sakamoto, J. et al. Pharmacokinetic study of weekly administration dose of paclitaxel in patients with advanced or recurrent gastric cancer in Japan. Gastric Cancer 10, 52–57 (2007). https://doi.org/10.1007/s10120-006-0411-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-006-0411-6