Abstract
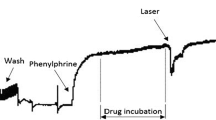
To evaluate whether the chronic effect of photobiomodulation therapy (PBM) on systolic arterial pressure (SAP) from two kidneys one clip (2 K-1C) hypertension animal models can cause a hypotensive effect. Serum levels of nitric oxide were also analyzed and the assessment of lipid peroxidation of the thoracic aorta artery. Male Wistar rats were used. Hypertensive animals (2 K-1C) with Systolic arterial pressure (SAP) greater than or equal to 160 mmHg were used. Systolic arterial pressure (SAP) was determined by the tail plethysmography technique. Normotensive (2 K) and hypertensive (2 K-1C) rats were treated to PBM for 4 weeks using a laser whose irradiation parameters were: red wavelength (λ) = 660 nm: operating continuously; 56 s per point (3 points) spot size = 0.0295 cm2; average optical power of 100 mW; energy of 5.6 J per point; irradiance of 3.40 W/cm2; fluency of 190 J/cm2 per point. The application was on the animals tails, at 3 different points simultaneously, in contact with the skin. To assess serum nitrite and nitrate (NOx) levels, blood collection was performed after chronic PBM treatment, 24 h after the last laser application. The evaluation of the lipid peroxidation of the thoracic aorta artery was performed by measuring the concentration of hydroperoxide by the FOX method. Chronic photobiomodulation therapy (PBM) by red laser (660 nm) can induce a hypotensive effect in 64% of 2 K-1C hypertensive animals, which we say responsive animals. There was no difference in serum NO levels 24 h after the last red laser application, between treated and non-treated groups. Aortic rings from 2 K-1C hypertensive animals present a higher lipid peroxidation. The chronic PBM treatment by red laser decreased aortic rings lipid peroxidation in hypertensive responsive groups, compared to control. our results indicate that chronic PBM made by red laser has an important hypotensive effect in renovascular hypertensive models, by a mechanism that involves decrease in oxidative stress from vascular beds.





Similar content being viewed by others
References
Malachias MVB, Ferreira S Filho, Souza WKSB, Ribeiro JM, Miranda RD, Jardim TSV (2016) 7th Brazilian Guideline of Arterial Hypertension: Chapter 11 - Arterial Hypertension in the elderly. Arq Bras Cardiol. 107(3 Suppl 3):64–66. https://doi.org/10.5935/abc.20160161
Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, Flack JM, Carter BL, Materson BJ, Ram CV, Cohen DL, Cadet JC, Jean-Charles RR, Taler S, Kountz D, Townsend R, Chalmers J, Ramirez AJ, Bakris GL, Wang J, Schutte AE, Bisognano JD, Touyz RM, Sica D, Harrap SB (2014) Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens 32(1):3–15. https://doi.org/10.1097/HJH.0000000000000065
Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K (2007) The burden and costs of chronic diseases in low-income and middle-income countries. Lancet 8 370(9603):1929–38. https://doi.org/10.1016/S0140-6736(07)61696-1
Scala LC, Magalhães LB, Machado A (2015) Epidemiologia da hipertensão arterial sistêmica. In: Moreira SM, Paola AV; Sociedade Brasileira de Cardiologia. Livro Texto da Sociedade Brasileira de Cardiologia. 2ª. ed. São Paulo: Manole, p. 780–5
Sim JJ, Bhandari SK, Shi J et al (2013) Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin Proc 88:1099–1107. https://doi.org/10.1016/.mayocp.2013.06.01719
Moncada S, Palmer RM, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43(2):109–142 (PMID: 1852778)
Rees DD, Palmer RM, Moncada S (1989) Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A 86(9):3375–3378. https://doi.org/10.1073/pnas.86.9.3375
Whittle BJ (1995) Nitric oxide in physiology and pathology. Histochem J 27(10):727–737
Pereira AC, Paulo M, Araújo AV, Rodrigues GJ, Bendhack LM (2011) Nitric oxide synthesis and biological functions of nitric oxide released from ruthenium compounds. Braz J Med Biol Res 44(9):947–957. https://doi.org/10.1590/s0100-879x2011007500084.
Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T (2001) Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104(22):2673–2678. https://doi.org/10.1161/hc4601.099485.Erratum.In:Circulation.2003Jul29;108(4):500
Yang O, Li J, Kong J (2015) The Endothelium as a Target for the Treatment of Heart Failure. Cell Biochem Biophys 72(3):751–756. https://doi.org/10.1007/s12013-015-0526-7
Cosentino F, Sill JC, Katusic ZS (1994) Role of superoxide anions in the mediation of endothelium-dependent contractions. Hypertension 23:229–235
Radi R, Beckman JS, Bush KM, Freeman BA (1991) Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288(2):481–7. https://doi.org/10.1016/0003-9861(91)90224-7
Hamblin MR (2017) Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys 4(3):337–361
Furchgott RF, Ehrreich SJ, Greenblatt E (1961) The photoactivated relaxation of smooth muscle of rabbit aorta. J Gen Physiol 44(3):499–519. https://doi.org/10.1085/jgp.44.3.499.PMID:13702637;PMCID:PMC2195116
Kubaszewski E, Peters A, McClain S, Bohr D, Malinski T (1994) Light-activated release of nitric oxide from vascular smooth muscle of normotensive and hypertensive rats. Biochem Biophys Res Commun 200(1):213–218. https://doi.org/10.1006/bbrc.1994.1436
Oishi JC et al (2017) Hypotensive acute effect of photobiomodulation therapy on hypertensive rats. Life Sci 178:56–60
De Moraes TF, Conceição-Filho JC, Oishi JC, Almeida-Lopes L, Parizotto NA, Rodrigues GJ (2019) Energy-dependent effect trial of photobiomodulation on blood pressure in hypertensive rats. Lasers Med Sci. https://doi.org/10.1007/s10103-019-02883-5
Goldblatt H, Lynch J, Hanzal RF, W.W, (1934) Summerville, studies on experimental hypertension I. the production of persistent elevation ofsystolic blood pressure by means of renal ischemia. J Exp Med 59(3):347–37
Shaffemburg CAD (1959) Devide to control constriction of main renal artery for production of hypertension in small animals. Proceed Soc Biol Med 101:676–677
Rodrigues GJ, Restini Carolina B A, Lunardi Claure N, Dos Anjos Mário, Neto Jorge E, Moreira Lusiane M, Bendhack (2010) Decreased number of caveolae in endothelial cells impairs the relaxation induced by acetylcholine in hypertensive rat aortas. Eur J Pharm 627:251–257
Rodrigues GJ, Lunardi CN, Lima RG, Santos CX, Laurindo FRM, da Silva RS, Bendhack LM (2008) Vitamin C improves the effect of a new nitric oxide donor on the vascular smooth muscle from renal hypertensive rats. Nitric Oxide 18:176–183
Archer S (1993) Measurement of nitric oxide in biological models. FASEB J 7:349–360
Jiang Z-Y, Woollard ACS, Wolff SP (1991) Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison TBA Assay An Iodomet Method Lip 26:853–856
Albertini R et al (2004) Effects of different protocol doses of low Power gallium–aluminum–arsenate (Ga–Al–As) laser radiation (650 nm) on carrageenan induced rat paw oedema. Journal of Photochemistry and Photobiology 74:101–107
Mezawa S, Iwata K, Naito K, Kamogawa H (1988) The possible analgesic effect of soft-laser irradiation on heat nociceptors in the cat tongue. Arch Oral Bio 33:693–694
Vinck EM, Cagnie BJ, CornelissenMJ DHA, Cambier DC (2003) Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med Sci 18:95–99. https://doi.org/10.1007/s10103-003-0262-x
Karu T (1999) Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B 49(1):1–17. https://doi.org/10.1016/S1011-1344(98)00219-X
Tomimura S, Silva BP, Sanches IC, Canal M, Consolim-Colombo F, Conti FF, De Angelis K, Chavantes MC (2014) Hemodynamic effect of laser therapy in spontaneously hypertensive rats. Arq Bras Cardiol 103(2):161–164. https://doi.org/10.5935/abc.20140117
Buzinari TC, Oishi JC, De Moraes TF, Vatanabe IP, Araújo HSS, Pestana CR, Rodrigues GJ (2017) Treatment with sodium nitroprusside improves the endothelial function in aortic rings with endothelial dysfunction. Eur J Pharm Sci 105:144–149. https://doi.org/10.1016/j.ejps.2017.04.022
Oishi JC, Castro CA, Silva KA, Fabricio V, Cárnio EC et al (2018) Endothelial Dysfunction and Inflammation Precedes Elevations in Blood Pressure Induced by a High-Fat Diet. Arq Bras Cardiol 110(6):558–567. https://doi.org/10.5935/abc.20180086
Sousa LE, Del Favero IF, Bezerra FS, Souza ABF et al. (2018) Environmental Enrichment Promotes Antioxidant Effect in the Ventrolateral Medulla and Kidney of Renovascular Hypertensive Rats. Manuscript received August 31, https://doi.org/10.5935/abc.20190166
Vaziri ND, Ni Z, Oveisi F (1998) Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension 31:1248–1254
Buzinari TC, De Moraes TF, Cárnio EC, Almeida-Lopes L, Salgado HC, Rodrigues GJ (2020) Photobiomodulation induces hypotensive effect in spontaneously hypertensive rats. Lasers Med Sci 35:567–572. https://doi.org/10.1007/s10103-019-02849-7
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R (2002) Prospective studies collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360(9349):1903–13. https://doi.org/10.1016/s0140-6736(02)11911-8
Siddiqui M, Dudenbostel T, Calhoun DA (2016) Resistant and Refractory Hypertension: Antihypertensive Treatment Resistance vs Treatment Failure. Can J Cardiol 32:603–606. https://doi.org/10.1016/j.cjca.2015.06.033
Acknowledgements
Special thanks to Professor Evelin Capellari Cárnio from Department of Nursing, general and specialized, Nursing School of Ribeirão Preto, University of São Paulo – USP, Ribeirão Preto, SP, Brazil for auxiliary the experiments of Serum Nitrite and Nitrate quantification with the equipment NO Analyzer 280i.
Author information
Authors and Affiliations
Contributions
MS and GR: Conceptualization; MS, TM and JN: Data curation; LM, TB and GR: Formal analysis; GR: Funding acquisition; MS: Investigation; GR: Methodology; GR: Project administration; GR: Resources; GR: Supervision; GR: Validation; GR: Visualization; LM, TB and GR: Roles/Writing—original draft; LM and GR: Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
dos Santos Carvalho Schiavon, M., de Moraes, L.H.O., de Moraes, T.F. et al. Chronic red laser treatment induces hypotensive effect in two-kidney one-clip model of renovascular hypertension in rat. Lasers Med Sci 38, 252 (2023). https://doi.org/10.1007/s10103-023-03918-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-023-03918-8