Abstract
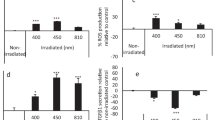
The aim of this study was to assess the ability of red light emitting diodes (LED) to modulate oxidative stress in human dental pulp fibroblasts (HDPFs) when different irradiation parameters are employed. Cells from primary teeth were seeded (100,000 cells/well) in 24-well plates in culture medium (DMEM). At 24 h after incubation, the culture medium was replaced with DMEM containing 10 μg/mL lipopolysaccharide (LPS). Thereafter, the cells were irradiated (LED 630 nm, 0.04 W/cm2 and 0.08 W/cm2) at 0 J/cm2 (control group), 4 J/cm2, 15 J/cm2, and 30 J/cm2; and their viability (MTT assay), number (Trypan Blue), synthesis of nitric oxide (NO) (Griess reagent), and reactive oxygen species (ROS) (fluorescence probe, DCFH-DA) were assessed. The Kruskal-Wallis and Mann-Whitney statistical tests using Bonferroni correction were employed (significance level of 5%). Compared to that in control fibroblasts, increased viability was observed in HDPFs exposed to LPS and irradiated with 15 J/cm2 and 30 J/cm2 at 0.04 W/cm2 and 4 J/cm2 and 15 J/cm2 at 0.08 W/cm2 (p < 0.05). Exposure to 4 J/cm2 at 0.04 W/cm2 and 15 J/cm2 and 30 J/cm2 at 0.08 W/cm2 modulated the oxidative stress in cells relative to that observed in non-irradiated LPS-treated pulp cells (p < 0.05). It was concluded that the irradiation strategies of using red LED with radiant exposures of 15 J/cm2 and 30 J/cm2 at 0.04 W/cm2 and 15 J/cm2 at 0.08 W/cm2 were the best parameters to decrease NO and ROS concentration and to stimulate viability of HDPFs exposed to LPS challenge.




Similar content being viewed by others
References
de Souza Costa CA, Hebling J, Scheffel DL, Soares DG, Basso FG, Ribeiro AP (2014) Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques. Dent Mater 30(7):769–784. https://doi.org/10.1016/j.dental.2014.04.010
Turrioni AP, Basso FG, Alonso JR, de Oliveira CF, Hebling J, Bagnato VS, de Souza Costa CA (2015) Transdentinal cell photobiomodulation using different wavelengths. Oper Dent 40(1):102–111. https://doi.org/10.2341/13-370-L
Peres MA, Daly B, Guarnizo-Herreño CC, Benzian H, Watt RG (2020) Oral diseases: a global public health challenge - authors' reply. Lancet 18;395(10219):186-187. https://doi.org/10.1016/S0140-6736(19)32997-6
Listl S, Galloway J, Mossey PA, Marcenes W (2015) Global economic impact of dental diseases. J Dent Res 94(10):1355–1361. https://doi.org/10.1177/0022034515602879
Schwendicke F, Meyer-Lueckel H, Stolpe M, Dörfer CE, Paris S (2014) Costs and effectiveness of treatment alternatives for proximal caries lesions. PLoS One 9(1):e86992. https://doi.org/10.1371/journal.pone.0086992
Fukuzaki Y, Shin H, Kawai HD, Yamanoha B, Kogure S (2015) 532nm low-power laser irradiation facilitates the migration of GABAergic neural stem/progenitor cells in mouse neocortex. PLoS One 10(4):1–14. https://doi.org/10.1371/journal.pone.0123833
Ballini A, Mastrangelo F, Gastaldi G, Tettamanti L, Bukvic N, Cantore S, Cocco T, Saini R, Desiate A, Gherlone E, Scacco S (2015) Osteogenic differentiation and gene expression of dental pulp stem cells under low-level laser irradiation: a good promise for tissue engineering. J Biol Regul Homeost Agents 29(4):813–822
Cotler HB (2015) The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop Rheumatol 2(5):00068. https://doi.org/10.15406/mojor.2015.02.00068
Brassolatti P, Bossini PS, Oliveira MC, Kido HW, Tim CR, Almeida-Lopes L, De Avó LR, Araújo-Moreira FM, Parizotto NA (2016) Comparative effects of two different doses of low-level laser therapy on wound healing third-degree burns in rats. Microsc Res Tech 79(4):313–320. https://doi.org/10.1002/jemt.22632
Uloopi KS, Vinay C, Ratnaditya A, Gopal AS, Mrudula KJ, Rao RC (2016) Clinical evaluation of low level diode laser application for primary teeth pulpotomy. J Clin Diagn Res 10(1):67–70. https://doi.org/10.7860/JCDR/2016/13218.7140
Bublitz C, Medalha C, Oliveira P, Assis L, Milares LP, Fernandes KR, Tim CR, Vasilceac FA, Mattiello SM, Renno AC (2014) Low-level laser therapy prevents degenerative morphological changes in an experimental model of anterior cruciate ligament transection in rats. Lasers Med Sci 29(5):1669–1678. https://doi.org/10.1007/s10103-014-1546-z
Zagatto AM, de Paula RS, Nakamura FY, de Lira FS, Lopes-Martins RÁ, de Paiva Carvalho RL (2016) Effects of low-level laser therapy on performance, inflammatory markers, and muscle damage in young water polo athletes: a double-blind, randomized, placebo-controlled study. Lasers Med Sci 31(3):511–521. https://doi.org/10.1007/s10103-016-1875-1
Dong T, Zhang Q, Hamblin MR, Wu MX (2015) Low-level light in combination with metabolic modulators for effective therapy of injured brain. J Cereb Blood Flow Metab 35(9):1435–1444. https://doi.org/10.1038/jcbfm.2015.87
Sotoudeh A, Jahanshahi A, Zareiy S, Darvishi M, Roodbari N, Bazzazan A (2015) The influence of low-level laser irradiation on spinal cord injuries following ischemia–reperfusion in rats. Acta Cir Bras 30(9):611–616. https://doi.org/10.1590/S0102-865020150090000005
Mostafavinia A, Masteri Farahani R, Abbasian M, Vasheghani Farahani M, Fridoni M, Zandpazandi S, Ghoreishi SK, Abdollahifar MA, Pouriran R, Bayat M (2015) Effect of pulsed wave low-level laser therapy on tibial complete osteotomy model of fracture healing with an intramedullary fixation. Iran Red Crescent Med J 17(12):e32076. https://doi.org/10.5812/ircmj.32076
Tim CR, Bossini PS, Kido HW, Malavazi I, von Zeska Kress MR, Carazzolle MF, Parizotto NA, Rennó AC (2016) Effects of low level laser therapy on inflammatory and angiogenic gene expression during the process of bone healing: a microarray analysis. J Photochem Photobiol B Biol 154:8–15. https://doi.org/10.1016/j.jphotobiol.2015.10.028
Marques NC, Neto NL, Rodini Cde O, Fernandes AP, Sakai VT, Machado MA, Oliveira TM (2015) Low-level laser therapy as an alternative for pulpotomy in human primary teeth. Lasers Med Sci 30(7):1815–1822. https://doi.org/10.1007/s10103-014-1656-7
Alonso JR, Turrioni AP, Basso FG, de Souza Costa CA, Hebling J (2016) Synthesis of dental matrix proteins and viability of odontoblast-like cells irradiated with blue LED. Lasers Med Sci 31(3):523–530. https://doi.org/10.1007/s10103-016-1889-8
Ferreira AN, Silveira L, Genovese WJ, de Araújo VC, Frigo L, de Mesquita RA, Guedes E (2006) Effect of GaAIAs laser on reactional dentinogenesis induction in human teeth. Photomed Laser Surg 24(3):358–365. https://doi.org/10.1089/pho.2006.24.358
Oliveira CF, Basso FG, Lins EC, Kurachi C, Hebling J, Bagnsto VS, de Souza Costa CA (2011) In vitro effect of low-level laser on odontoblast-like cells. Laser Phys Lett 8(2):155–163. https://doi.org/10.1002/lapl.201010101
Holder MJ, Milward MR, Palin WM, Hadis MA, Cooper PR (2012) Effects of red light-emitting diode irradiation on dental pulp cells. J Dent Res 91(10):961–966. https://doi.org/10.1177/0022034512456040
Turrioni AP, Basso FG, Montoro LA, Almeida Lde F, Costa CA, Hebling J (2014) Phototherapy up-regulates dentin matrix proteins expression and synthesis by stem cells from human-exfoliated deciduous teeth. J Dent 42(10):1292–1299. https://doi.org/10.1016/j.jdent.2014.07.014
Turrioni AP, Montoro LA, Basso FG, de Almeida LF, Costa CA, Hebling J (2015) Dose-responses of stem cells from human exfoliated teeth to infrared LED irradiation. Braz Dent J 26(4):409–415. https://doi.org/10.1590/0103-6440201300148
Seo YK, Park JK, Song C, Kwo SY (2013) Comparison of light-emitting diode wavelength on activity and migration of rabbit ACL cells. Lasers Med Sci 29(1):245–255. https://doi.org/10.1007/s10103-013-1322-5
Nishioka MA, Pinfildi CE, Sheliga TR, Arias VE, Gomes HC, Ferreira LM (2012) LED (660 nm) and laser (670 nm) use on skin flap viability: angiogenesis and mast cells on transition line. Lasers Med Sci 27(5):1045–1050. https://doi.org/10.1007/s10103-011-1042-7
Turrioni APS, Alonso JRL, Basso FG, Moriyama LT, Hebling J, Bagnato VS, De Souza Costa CA (2013) LED light attenuation through human dentin: a first step toward pulp photobiomodulation after cavity preparation. Am J Dent 26(6):319–323
Tziafas D, Kodonas K (2010) Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod 36(5):781–789. https://doi.org/10.1016/j.joen.2010.02.006
Bletsa A, Berggreen E, Fristad I, Tenstad O, Wiig H (2006) Cytokine signalling in rat pulp interstitial fluid and transcapillary fluid exchange during lipopolysaccharide-induced acute inflammation. J Physiol 573(Pt 1):225–236. https://doi.org/10.1113/jphysiol.2006.104711
Kendall HK, Marshall RI, Bartold PM (2001) Nitric oxide and tissue destruction. Oral Dis 7(1):2–10
Montoro LA, Turrioni AP, Basso FG, de Souza Costa CA, Hebling J (2014) Infrared LED irradiation photobiomodulation of oxidative stress in human dental pulp cells. Int Endod J 47(8):747–755. https://doi.org/10.1111/iej.12211
Hadis MA, Zainal SA, Holder MJ, Carroll JD, Cooper PR, Milward MR, Palin WM (2016) The dark art of light measurement: accurate radiometry for low-level light therapy. Lasers Med Sci 31(4):789–809. https://doi.org/10.1007/s10103-016-1914-y
Karu TI (2010) Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 62(8):607–610. https://doi.org/10.1002/iub.359
de Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22(3):7000417. https://doi.org/10.1109/JSTQE.2016.2561201
Wang X, Tian F, Soni SS, Gonzalez-Lima F, Liu H (2016) Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep 3(6):30540. https://doi.org/10.1038/srep30540
El Karim IA, Linden GJ, Curtis TM, About I, McGahon MK, Irwin CR, Killough SA (2011) Lundy FT (2011) Human dental pulp fibroblasts express the "cold-sensing" transient receptor potential channels TRPA1 and TRPM8. J Endod 37(4):473–478. https://doi.org/10.1016/j.joen.2010.12.017
Wei L, Chen Y, Zhang C, Liu M, Xiong H (2019) Leptin induces IL-6 and IL-8 expression through leptin receptor Ob-Rb in human dental pulp fibroblasts. Acta Odontol Scand 77(3):205–212. https://doi.org/10.1080/00016357.2018.1536280
Wang D, Sun S, Xue Y, Qiu J, Ye T, Zhang R, Song B, He W, Zhang Y, Jiang W (2021) MicroRNA-223 negatively regulates LPS-induced inflammatory responses by targeting NLRP3 in human dental pulp fibroblasts. Int Endod J 54(2):241–254. https://doi.org/10.1111/iej.13413
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and invivo. Proc Natl Acad Sci 97(25):13625–13630. https://doi.org/10.1073/pnas.240309797
Almeida Lde F, Turrioni AP, Basso FG, Montoro LA, Souza-Costa CA, Hebling J (2016) Red LED photobiomodulates the metabolic activity of odontoblast-like cells. Braz Dent J 27(4):375–380. https://doi.org/10.1590/0103-6440201600152
Turrioni AP, Basso FG, Montoro LA, Almeida LFD, de Souza Costa CA, Hebling J (2017) Transdentinal photobiostimulation of stem cells from human exfoliated primary teeth. Int Endod J 50(6):549–559. https://doi.org/10.1111/iej.12665
Tagliani MM, Oliveira CF, Lins EMM, Kurachi C, Hebling J, Bagnato VS, de Souza Costa CA (2010) Nutritional stress enhances cell viability of odontoblast-like cells subjected to low level laser irradiation. Laser Phys Lett 7(3):247–251. https://doi.org/10.1002/lapl.200910137
Stashenko P, Teles R, D’Souza R (1998) Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med 9(4):498–521. https://doi.org/10.1177/10454411980090040701
Chang J, Zhang C, Tani-Ishii N, Shi S, Wang CY (2005) NF-κB activation in human dental pulp stem cells by TNF and LPS. J Dent Res 84(11):994–998. https://doi.org/10.1177/154405910508401105
Basso FG, Soares DG, Pansani TN, Turrioni AP, Scheffel DL, de Souza Costa CA, Hebling J (2015) Effect of LPS treatment on the viability and chemokine synthesis by epithelial cells and gingival fibroblasts. Arch Oral Biol 60(8):1117–1121. https://doi.org/10.1016/j.archoralbio.2015.04.010
Jafari M, Ansari-Pour N (2019) Why, when and how to adjust your P values? Cell J 20(4):604–607. https://doi.org/10.22074/cellj.2019.5992
Mowbray M, McLintock S, Weerakoon R, Lomatschinsky N, Jones S, Rossi AG, Weller RB (2009) Enzyme-independent NO stores in human skin: quantification and influence of UV radiation. J Invest Dermatol 129(4):834–842. https://doi.org/10.1038/jid.2008.296
Huang YY, Chen ACH, Carroll JD, Hamblin MR (2009) Biphasic dose response in low level lightherapy. Dose-Response 7(4):358–383. https://doi.org/10.2203/dose-response.09-027.Hamblin
Borutaite V, Budriunaite A, Brown GC (2000) Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim Biophys Acta Bioenerg 1459(2-3):405–412. https://doi.org/10.1016/S0005-2728(00)00178-X
Liew FY, Cox FE (1991) Nonspecific defense mechanism: the role of nitric oxide. Immunol Today 12(3):17–21. https://doi.org/10.1016/S0167-5699(05)80006-4
Szabo C (1995) Alterations in nitric oxide production in various forms of circulatory shock. New Horizons Sci Pract Acute Med 3(1):2–32
de Oliveira Duque CC, Soares DG, Basso FG, Hebling J, de Souza Costa CA (2017) Influence of enamel/dentin thickness on the toxic and esthetic effects of experimental in-office bleaching protocols. Clin Oral Investig 21(8):2509–2520. https://doi.org/10.1007/s00784-017-2049-7
Bordini EAF, Cassiano FB, Silva ISP, Usberti FR, Anovazzi G, Pacheco LE, Pansani TN, Leite ML, Hebling J, de Souza Costa CA, Soares DG (2020) Synergistic potential of 1α,25-dihydroxyvitamin D3 and calcium–aluminate–chitosan scaffolds with dental pulp cells. Clin Oral Investig 24(2):663–674. https://doi.org/10.1007/s00784-019-02906-z
Funding
The authors are grateful for funding from the following Brazilian agencies: Fundação de Amparo à Pesquisa de Minas Gerais - Brazil (FAPEMIG Universal, APQ00315-16), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil) (CNPq Universal, 434204-2018-8), and the Coordenação de Aperfeiçoamento de Pessoal no Ensino Superior (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Institutional Research Ethics Committee of the Faculty of Dentistry of the Federal University of Uberlândia (process 54488816.2.0000.5152).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bonvicini, J.F.S., Basso, F.G., de Souza Costa, C.A. et al. Photobiomodulation effect of red LED (630 nm) on the free radical levels produced by pulp cells under stress conditions. Lasers Med Sci 37, 607–617 (2022). https://doi.org/10.1007/s10103-021-03309-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03309-x