Abstract
Fungal biofilm has remained a serious medical problem that complicates treatment of mycoses. In particular, once biofilms are formed, they display high levels of resistance against most common antifungals. Candida auris is currently considered as a serious emerging fungal pathogen frequently exhibiting high levels of resistance to antifungals. Recent studies have confirmed that C. auris shares similarity with Candida albicans in regards to virulence-associated proteins involved in adherence and biofilm development. Complement receptor 3-related protein (CR3-RP) is one of the key surface antigens expressed by Candida species during biofilm formation. Here, we have investigated the presence of this cell surface moiety on the surface of C. auris, as well as the potential of anti-CR3-RP polyclonal antibody (Ab) to inhibit biofilm formation by this emerging fungal pathogen. Using indirect immunofluorescence and ELISA, we were able to confirm the presence of CR3-RP in C. auris cells within biofilms. Further, not only anti-CR3-RP Ab was able to inhibit biofilm formation by multiple C. auris strains when added during the adherence phase, but it also demonstrated activity against C. auris 24-h pre-formed biofilms, which compared favorably to levels of inhibition achieved by treatment with current conventional antifungals fluconazole, amphotericin B, and caspofungin. Overall, our data demonstrate the presence of this antigen on the surface of C. auris and points to the potential of anti-CR3-RP Ab in eradication of biofilms formed by this novel fungal pathogen.
Similar content being viewed by others
Introduction
Complement receptor 3-related protein (CR3-RP) is an immunogenic surface protein expressed on the cell wall of Candida species (spp.) during adherence and biofilm formation, with functional and structural similarity to the human complement receptor 3 (CR3) expressed on neutrophils, macrophages, and monocytes [1,2,3,4]. Recently, our group has demonstrated an important role of CR3-RP in adherence and biofilm development of different Candida spp. Anti-adhesive and anti-biofilm effects of anti-CR3-RP polyclonal antibody (Ab) directed against the antigen CR3-RP on Candida cell wall have already been described [5,6,7]. Most recently, a reduction in adherence and biofilm formation was proven not only in Candida albicans, but also in Candida dubliniensis after treatment of the yeast cells with anti-CR3-RP Ab in vitro/ex vivo and in vivo [8].
Candida auris is considered an emerging fungal pathogen, spreading rapidly around the world, and increasingly associated with nosocomial invasive fungal infections which very often are resistant to treatment with current conventional antifungals [9,10,11,12]. It has already been proven that this species (sp.) is able to colonize substrates and subsequently form biofilms in vitro and in vivo [12,13,14]. The genome of C. auris contains genes encoding for two types of efflux pumps, ABC transporters and major facilitators [15, 16], which could explain why C. auris often displays multidrug resistance to various antifungals [17, 18]. Moreover, C. auris expresses many genes encoding virulence-associated proteins similar to those expressed in C. albicans, some of which have been associated with invasion and biofilm development [17]. CR3-RP is believed to be one of the important surface antigens involved in virulence-associated processes. Beside C. albicans and C. dubliniensis, this protein was also identified in C. parapsilosis, C. glabrata, and C. krusei [2, 8]. Antibodies directed against surface antigens are considered to be potential candidates for the development of novel approaches for the therapy of Candida infections [19,20,21]. In the case of anti-CR3-RP Ab, its potential anti-adherence and anti-biofilm effect have already been described in C. albicans and C. dubliniensis by our group [7, 8]. Mentioned results were a motivation for continuation in this research using C. auris. Thus, the main aims of this research were to investigate the presence of CR3-RP on the surface of C. auris and to evaluate the potential of anti-CR3-RP Ab in eradication of multidrug-resistant C. auris biofilms.
Material and methods
Candida strains and growth conditions
C. albicans wild-type strain SC5314 [22] was used as control in all experiments. The C. auris clinical isolates 0390, 0383, and 0386 with different patterns of resistance to antifungals were obtained from the Centers for Diseases Control and Prevention (CDC) Antimicrobial resistance bank (USA). Prior to all experiments, cells from stocks stored at − 80 °C were streaked onto a yeast extract-peptone-dextrose plate (YPD) supplemented with 2% of agar (Becton Dickinson, USA) and incubated overnight at 30 °C. One loopful of cells from YPD agar plates was inoculated into flasks containing 20 ml of YPD broth and grown in an orbital shaker (180 rpm, New Brunswick Scientific, USA), for up to 16 h at 30 °C. The cells were then washed twice with phosphate-buffered saline (PBS) buffer (Sigma, USA) and adjusted to the appropriate density for each experiment.
Antifungal susceptibility of biofilms formed by C. albicans and C. auris strains
All strains were tested for their ability to form biofilm (Supplementary Material Fig. S1). The susceptibility of biofilms against fluconazole (FLU, SAGENT Pharmaceuticals, USA), caspofungin (CAS, Merck, USA), and amphotericin B (AMB, Sigma, USA) was tested in vitro following the 96-well microtiter plate method first described by Ramage et al. [23] with slight modifications. The initial inoculum was adjusted to 2 × 106 cells per ml in RPMI 1640 medium containing 2% D-glucose (Corning, USA) and buffered with 165 mM morpholine propanesulfonic acid (MOPS; Sigma, USA) to pH 7.0. The anti-biofilm activity of the antifungals was tested in two different modalities: (1) agents were added at the beginning of biofilm formation and (2) antifungal drugs were added to 24-h pre-mature biofilms. In both treatment modalities, the plates were washed after incubation, and the viability of cells within the biofilms was evaluated using an XTT (2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-2Htetrazolium-5-carboxanilide sodium salt, Sigma, USA) reduction assay as previously described [23]. Results were calculated as a mean value ± standard deviation (SD) from at least five parallel wells and from two independent experiments. Each experiment contained positive control (biofilm without drug, to allow for uninterrupted biofilm formation) and negative control (no cells, to monitor contamination and to be able to calculate the percent inhibition). The extent of biofilm inhibition was calculated as a percentage of colorimetric readings of biofilm cells in treated wells and compared to the control sample without agents, which was set to 100%. From these values, the “sessile minimum inhibitory concentrations” (SMICs) was calculated at 50% (for FLU and CAS) and 100% inhibition (for AMB) as per Ramage et al. [23].
Isolation and quantification of CR3-RP antigen in C. auris protein lysates
The presence of CR3-RP was determined in biofilm cells of C. auris using indirect immunofluorescence, and levels of expression of CR3-RP were quantified using an ELISA assay (enzyme-linked immunosorbent assay), according to the protocols previously published by our group [8, 24] with some modifications. For this work, one representative C. auris strain (C. auris 0390) was selected based on biofilm production and resistance profile. The strain C. albicans SC5314 was used as the control. Fluorescence was detected by inverted fluorescence microscope ZEISS Axio Observer 5 (Zeiss, Germany) (excitation/emission spectra 485/515 nm). The pictures were captured by ZEISS Axiocam 503 (Zeiss, Germany) and evaluated by software ZEN Pro (Zeiss, Germany). The level of CR3-RP was quantified in protein lysates of the tested strains using an ELISA assay as previously described by Chupáčová et al. [8]. Proteins were extracted from (i) planktonic cells from overnight yeast suspension, (ii) biofilm cells after 24-h cultivation, and (iii) biofilm cells after 48-h cultivation. Results were calculated as a mean value ± SD from at least five parallel wells and two independent experiments.
Anti-biofilm activity of anti-CR3-RP Ab
The inhibitory effect of anti-CR3-RP Ab in biofilm formation as well as its activity against preformed biofilms was tested following mostly the same protocol described above for antifungal susceptibility testing [23], but the diluted anti-CR3-RP Ab was tested instead of antifungal agents. For inhibition of biofilm formation, 100 μl of anti-CR3-RP Ab (dilution 1:50 in fresh RPMI-MOPS) was added to the wells, and plates were incubated for 90 min at 37 °C. After the adherence phase, non-adhered cells were removed and adherent cells were washed twice with PBS. Adherent cells were then overlaid again with 100 μl of anti-CR3-RP Ab and incubated at 37 °C for a further 48 h. For activity against 24-h pre-formed biofilms, 100 μl of the diluted anti-CR3-RP Ab was added to wells. Plates were incubated for another 24 h at 37 °C. The XTT reduction assay was performed as described above. Each experiment was performed in five parallel wells and performed twice. Data were expressed as mean values ± SD.
Microscopy
For microscopy, biofilms of C. auris 0390 and C. albicans SC5314 were prepared using a similar protocol as above, but instead using 24-well microtiter plates (Corning Inc., USA) with a final volume of 500 μl in each well. The final dilution of anti-CR3-RP Ab was 1:100 in RPMI-MOPS and Ab was added to the cells at t = 0 h (for inhibition of biofilm formation) and t = 24 h (for activity against pre-formed biofilms). RPMI-MOPS medium without Ab was used as a control. After 48 h, the medium was aspirated, the biofilms were gently washed with PBS and stained with crystal violet solution (0.6 g crystal violet, Sigma, USA; prepared in 10 ml of isopropanol, 10 ml of methanol, and 180 ml of Millipore water). After 5 min, the crystal violet was removed and plates were washed twice with 500 μl of distilled water. Samples were directly observed using a 40× objective in an inverted system microscope (Westover Scientific, USA) equipped for photography. The images were processed for display using Micron software (Westover Scientific, USA).
Statistical analyses
Results were evaluated by statistical analysis using one-way t test using Graph Pad Prism software (Graph Pad, San Diego, USA). Differences were considered statistically significant at p < 0.05 (∗), highly significant at p < 0.01 (∗∗), and extremely significant at p < 0.001 (∗∗∗).
Results and discussion
The anti-biofilm effect of anti-CR3-RP Ab has been described recently in some Candida spp. [7, 8] and this work represents a continuation of those experiments, with a focus on the effectiveness of Ab against the multidrug-resistant pathogen C. auris, in particular in biofilm formed in vitro.
Resistance of C. auris biofilms against commercial antifungal agents
Formation of fungal biofilms represents a major medical problem mostly due to their recalcitrance to antifungal treatment [25] as cells within the biofilms are up to 1000-fold more resistant compared to their non-biofilm, planktonic counterparts [23, 26, 27]. Our initial experiments demonstrated that all C. auris isolates tested were able to form biofilm in vitro, but to a lower extent than C. albicans (Supplementary Fig. S1). The results are similar to those reported by Sherry et al. [13] for a different C. auris strains. In agreement with mentioned study, our results confirmed the decreased susceptibility of C. auris biofilms to current antifungals FLU, CAS, and AMB (Table 1). When drugs were added at the beginning of biofilm formation during the adherence phase (t = 0 h), FLU was the least effective drug against all C. auris isolates (SMIC50 > 64 μg/ml). SMIC50 values for CAS were determined to be 0.5–1 μg/ml. Interestingly, C. auris 0390 also demonstrated a higher levels of resistance against AMB. When the antifungal drugs were added to the 24-h pre-formed biofilm, results clearly indicated the intrinsic resistance of biofilms formed by all three tested C. auris isolates (Table 1). These results confirmed that eradication of C. auris biofilms by conventional drugs is problematic and searching for alternative approaches seems to be essential.
C. auris expresses CR3-RP antigen
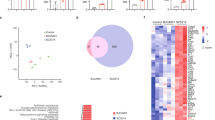
The expression of CR3-RP has already been observed in some Candida spp. [2, 8, 28]. Therefore at first, it was important to demonstrate whether CR3-RP is expressed by C. auris as well. As it is shown in Fig. 1, indirect immunofluorescence using the anti CR3-RP Ab demonstrated the expression of this antigen on the surface of C. auris cells, with fluorescence levels comparable to those observed for the C. albicans control cells (Fig. 1a, d). The same anti-CR3-RP Ab was used in an ELISA assay in order to quantity levels of CR3-RP. As it is also seen in Fig. 1g, results of this ELISA corroborated the presence of CR3-RP in C. auris lysate to similar level compared to C. albicans. Additionally, these results proved slightly increased quantity of CR3-RP in 24-h pre-matured biofilm compared to the planktonic cells for both C. albicans and C. auris; however, differences were not statistically significant. The image from indirect immunofluorescence also confirmed the presence of CR3-RP in biofilms of both spp. It was initially believed that the expression of CR3-RP was mainly associated with mycelial form [29], but a subsequent study confirmed the expression of this antigen in the yeast form as well [2]. Our results were in agreement with that observation; the presence of CR3-RP was confirmed in hyphae of C. albicans SC5314, but also in the yeast form of C. auris 0390. Thus, CR3-RP may represent an attractive target for prevention and treatment of infections associated with different Candida spp. including C. auris [18].
Expression of the CR3-RP antigen in cells from biofilms of C. albicans SC5314 (a) and C. auris 0390 (d) as visualized by indirect immunofluorescence using the anti-CR3-RP Ab. b, e Bright filed images; c, f negative controls without primary antibody. Magnification 400×. g Results of ELISA to quantity levels of CR3-RP in protein lysates of planktonic and biofilm cells of C. albicans SC5314 and C. auris 0390. Negative control—samples without Ab; positive control—synthetic peptide CR3-RP in concentration of 1 μg/ml
Activity of anti CR3-RP Ab against C. auris biofilms
Currently, fungal surface antigens are involved in the study of novel approaches for the treatment of candidiasis, including vaccine development [22,23,24, 30]. Taking into account this information, we were interested in examining the potential activity of anti-CR3-RP Ab against resistant C. auris biofilms. In this set of experiments, the effectiveness of anti-CR3-RP Ab on the C. auris biofilm compared to the activity of conventional antifungals was studied. Results summarized in Fig. 2 demonstrate that incubation of yeasts in the presence of anti-CR3-RP Ab resulted in higher inhibition of biofilm formation compared to all antifungal agents tested (FLU, CAS, and AMB) in both C. albicans (Fig. 2a) and C. auris isolates (Fig. 2b–d). For all tested isolates, treatment with anti-CR3-RP Ab resulted in statistically significant reduction in the metabolic activity of biofilm cells with 73% for C. auris 0390, 36% for C. auris 0383, and 49% for C. auris 0386, compared to the untreated controls. Inhibition of biofilm formation of the reference strain C. albicans SC5314 was 75%, also confirming results from our previous work [8]. These levels of biofilm inhibition compared favorable to those obtained with current antifungals. CAS was the only drug able to inhibit biofilm of the C. albicans strain and only one C. auris (0386) isolate by more than 50% (p < 0.001).
Activity of anti-CR3-RP Ab to inhibit biofilm formation by C. albicans SC5314 (a) and C. auris strains (bC. auris 0390; cC. auris 0383; dC. auris 0386). The anti-CR3-RP Ab (white columns) or antifungal drugs (gray columns) were added at t = 0 h in the adherence phase of biofilm formation. Cells within biofilms were significantly less metabolically active compared to the control after administration of Ab (p < 0.001 (***))
For the second modality treatment, anti-CR3-RP Ab was added to the 24-h pre-matured biofilms of C. auris and C. albicans. As it is shown in Fig. 3, treatment with anti-CR3-RP Ab resulted in statistically significant differences (p < 0.001) in metabolic activity of biofilm cells in all tested C. auris isolates, as well as in the control strain C. albicans SC5314l, although as expected, these decreases were lower than those observed for inhibition of biofilm formation, never reaching > 50% inhibition. However, the activity of anti-CR3-RP Ab was higher compared to all three conventional antifungals tested.
Activity of anti-CR3-RP Ab against pre-formed mature biofilms of C. albicans SC5314 (a) and C. auris strains (bC. auris 0390; cC. auris 0383; dC. auris 0386). The anti-CR3-RP Ab (white columns) or antifungal drugs (gray columns) were added at t = 24 h after biofilms of the different strains were fully formed. Cells within biofilms were significantly less metabolically active compared to the control after administration of Ab (p < 0.001 (***))
The anti-biofilm effect of anti-CR3-RP Ab was also supported by brightfield microscopy observations (Fig. 4). Results showed significant reduction in biofilm density and quantity for both tested strains of C. auris (0390) and C. albicans SC5314 (control) not only when anti-CR3-RP Ab was added at t = 0 h (Fig. 4b, e), but also when anti CR3-RP Ab was administered after 24 h to pre-formed biofilm (Fig. 4c, f). These observations were compared to the control biofilms developed in the absence of treatment (Fig. 4a, d).
Effect of anti-CR3-RP Ab on biofilms formed by C. albicans SC5314 (a–c) and C. auris 0390 (d–f) on polystyrene surface in vitro; control 48-h biofilm without antibody (a, d); biofilm formed in the presence of anti-CR3-RP Ab added in the adherence phase (t = 0 h) (b, e); biofilm formed in the presence of anti-CR3-RP Ab added to pre-formed biofilm (t = 24 h) (c, f). Bars represent 100 μm. All samples were stained with 0.6% crystal violet
The strategy in which Abs block target Candida surface proteins has been postulated in many studies as a promising alternative approach for combating Candida infection [22, 24, 30]. Here, after demonstrating the presence of CR3-RP antigen in the C. auris cell wall, we also confirmed the effectiveness of anti-CR3-RP Ab on multidrug-resistant biofilm formed by C. auris. The specific mechanism of action by which this Ab targets Candida spp., and in particular its activity against Candida biofilm, is unknown. It has been reported that anti-CR3-RP Ab can bind covalently to CR3-RP antigen in cell wall of Candida and through this mechanisms block the function of CR3-RP as a mimic protein, protecting the yeast against immune system reaction [7]. Possibility of cross reaction with other virulence proteins leading to decrease of adherence and biofilm formation could also be assumed. For example, Fujibayashi et al. (2009) described the blocking cell wall antigens involved in adhesion with 3 anti-Candida IgY Abs produced in egg yolk and suggested a possible cross-reaction of Abs with Als3 and Hwp1 proteins leading to reduction in adhesion and biofilm development [31]. We could also hypothesize that binding of anti-CR3-RP Ab to its corresponding CR3-RP antigen in yeasts may lead to changes in the integrity of the cell wall or in cell surface properties, which could explain, at least in part, its activity against biofilms. However, more experiments are needed to confirm this hypothesis. Overall, our results demonstrated the activity of CR3-RP Ab against multidrug-resistant C. auris biofilms and may serve as the basis for the development of alternative strategy to tackle these difficult to treat infections caused by C. auris.
References
Heidenreich F, Dierich MP (1985) Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun 50:598–600
Bujdáková H, Würzner R, Klobušický M, Dierich MP (1997) Expression and quantification of the iC3b-binding protein in different Candida albicans strains and their morphological stages. FEMS Immunol Medical Microbiol 18:147–152
Paulovičová E, Bystrický S, Machová E, Bujdáková H (2008) Immune responsiveness of a novel peptidoglycan conjugate prepared from surface Candida immunogens: mannan and CR3-related protein. FEMS Immunol Med Microbiol 53:421–428
Calderone RA (1995) Recognition of endothelial cells by Candida albicans: role of complement-binding proteins. Can J Bot 73:1154–1159
Hostetter MK (2008) The iC3b receptor of Candida albicans and its role in pathogenesis. Vaccine 26:108–1012
Bujdáková H, Paulovičová E, Borecká-Melkusová S et al (2008) Antibody response to the 45kDa Candida albicans antigen in an animal model and potential role of the antigen in adherence. J Med Microbiol 57:1466–1472
Bujdáková H, Paulovičová E, Paulovičová L, Šimová Z (2010) Participation of the Candida albicans surface antigen in adhesion, the first phase of biofilm development. FEMS Immunol Med Microbiol 59:485–492
Chupáčová J, Borhi E, Morace G et al (2018) Anti-biofilm activity of antibody directed against surface antigen CR3-RP - comparison of Candida albicans and Candida dubliniensis. FEMS Pathog Dis 76(1). https://doi.org/10.1093/femspd/ftx127
Satoh K, Makimura K, Hasumi Y et al (2009) Candida auris sp. nov., a novel novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 53:41–44
Chowdhary A, Sharma C, Meis JF (2017) Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 13(5):e1006290 https://doi.org/10.1371/journal.ppat.1006290
Dolande M, Garcia N, Capote AM et al (2017) Candida auris: antifungal multi-resistant emerging yeast. Curr Fungal Infect Rep 11:197–202
Larkin E, Hager C, Chandra J et al (2017) The emerging pathogen Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother 61:e02396–e02316. https://doi.org/10.1128/AAC.02396-16
Sherry L, Ramage G, Kean R et al (2017) Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg Infect Dis 23(2):328–331
Fakhim H, Vaezi A, Dannaoui E et al (2018) Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in murine model. Mycoses 2018:1–6
Chatterjee S, Alampalli S, Nageshan RK et al (2015) Draft genome of commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genomics 16:686
Sharma D, Kumar N, Pandey R et al (2016) Whole genome sequencing of emerging multidrug resistant Candida auris isolates in India demonstrates low genetic variation. New Microbes New Infect 13:77–82
Munoz JF, Gade L, Chow NA, et al (2018) Genomic basis of multidrug resistance mating and virulence in Candida auris and related emerging species. bioRxiv 299917. https://doi.org/10.1101/299917
Sears D, Schwartz BS (2017) Candida auris: an emerging multidrug-resistant pathogen. Int J Infect Dis 63:95–98
Coleman DA, Oh SH, Zhao X et al (2009) Monoclonal antibodies specific for Candida albicans ALS3 that immunolabel fungal cells in vitro and in vivo and block adhesion to host surfaces. J Microbiol Methods 78:71–78
Mishra NN, Ali S, Shukla PK (2015) A monoclonal antibody against 47.2 kDa cell surface antigen prevents adherence and affects biofilm formation of Candida albicans. World J Microbiol Biotechnol 31:11–21
Torosantucci A, Tumbarello M, Bromuro C et al (2017) Antibodies against a β-glucan-protein complex of Candida albicans and its potential as indicator of protective immunity in candidemic patients. Sci Rep 7:2722. https://doi.org/10.1038/s41598-017-02977-6
Gillum AM, Tsay EY, Kirsch DR (1984) Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198:179–182
Ramage G, Van de Walle K, Wickes BL, Lopez-Ribot JL (2001a) Standardized method for in vitro antifungal susceptibility testing of Candida albicans. Antimicrob Agents Chemother 45(9):2475–2479
Bujdáková H, Lell C, Gruber A et al (1999) The influence of subinhibitory concentrations of conventional and experimental antifungal drugs on the expression of the iC3b binding protein in Candida albicans strains during filamentation. Pathog Dis 26(1):1–10
Lohse MB, Gulati M, Johnson AD, Nobile CJ (2018) Development and regulation of single and multi-species Candida albicans biofilms. Nat Rev Microbiol 16(1):19–31
Ramage G, Vandewalle K, Wickes NL, López-Ribot JL (2001b) Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol 18:163–170
Ramage G, Rajendran R, Sherry L, Williams C (2012) Fungal biofilm resistance. Int J Microbiol 2012:528521
Paulovičová E, Bujdáková H, Chupáčová J et al (2015) Humoral immune response to Candida albicans complement receptor 3-related protein in the atopic subjects with vulvovaginal candidiasis. Novel sensitive marker for Candida infection. FEMS Yeast Res 15. https://doi.org/10.1093/femsyr/fou001
Alaei S, Larcher C, Ebenbichler C et al (1993) Isolation and biochemical characterization of the iC3b receptor of Candida albicans. Infect Immun 61:1395–1399
De Bernardis F, Amacker M, Arancia S et al (2012) A virosomal vaccine against candida vaginitis: immunogenicity, efficacy and safety profile in animal models. Vaccine 30(30):4490–4498
Fujibayashi T, Nakamura M, Tominaga A et al (2009) Effects of IgY against Candida albicans and Candida spp. adherence and biofilm formation. Jpn J Infect Dis 62:337–342
Acknowledgements
The authors wish to thank Daniel Montelongo-Jauregui, MSc. (The University of Texas at San Antonio, TX, USA) for help with performing fluorescence microscopy of indirect immunofluorescence.
Funding
This work was supported by the Slovak Research and Development Agency under contract no. [APVV-15-0347 to H. B.], and by the grant VEGA [1/0628/15 to H. B.] supported by the Ministry of Education, Science, Research and Sport of the Slovak Republic. This research was also supported by Fulbright Commission in Slovak Republic (J. D. was the Fulbright Visiting Student Researcher at J.L.L.R lab during period Sept 2017–Feb 2018). J.L.L.R acknowledges support by the Margaret Batts Tobin Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Electronic supplementary material
ESM 1
(DOCX 202 kb)
Rights and permissions
About this article
Cite this article
Dekkerová, J., Lopez-Ribot, J.L. & Bujdáková, H. Activity of anti-CR3-RP polyclonal antibody against biofilms formed by Candida auris, a multidrug-resistant emerging fungal pathogen. Eur J Clin Microbiol Infect Dis 38, 101–108 (2019). https://doi.org/10.1007/s10096-018-3400-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3400-x