Abstract
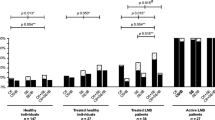
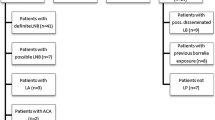
The diagnosis of Lyme disease is very complicated and a single diagnostic method cannot exclude infection. We assessed the performance of two commercially available Borrelia burgdorferi rapid diagnostic tests (RDT) in comparison to multiple laboratory-based diagnostic assays using specimens with a gradually increasing probability of Borrelia infection. Based on 200 specimens, the analytical sensitivities for IgG and IgM were 18 and 23 % for the Lyme RDT and 24 and 32 % for the Borreliose Complete RDT. The sensitivity for detecting diagnosed Lyme borreliosis cases was low (26 % Lyme RDT and 32 % with the Borreliose Complete RDT respectively), whereas the specificity was good (85 % Lyme RDT and 88 % Borreliose Complete). Based on this evaluation, the performance of RDTs in detecting Lyme borreliosis appeared to be below that of laboratory-based diagnostics.

Similar content being viewed by others
References
Stanek G, Wormser GP, Gray J, Strle F (2012) Lyme borreliosis. Lancet 379:461–473
Aguero-Rosenfeld M, Wang Q, Schwartz I, Wormser GP (2005) Diagnosis of Lyme borreliosis. Clin Microbiol Rev 18:484–509
Jaakola S, Lyytikäinen O, Rimhanen-Finne R et al (2013) Infectious diseases in Finland 2013. National Institute of Health and Welfare, Helsinki
Busson L, Reynders M, Van den Wijngaert S et al (2012) Evaluation of commercial screening tests and blot assays for the diagnosis of Lyme borreliosis. Diagn Microbiol Infect Dis 73:246–251
Lee SH, Vigliotti JS, Vigliotti VS, Jones W, Shearer DM (2014) Detection of borreliae in archived sera from patients with clinically suspect Lyme disease. Int J Mol Sci 15:4284–4298
Ang CW, Notermans DW, Hommes M, Simoons-Smit AM, Herremans T (2011) Large differences between test strategies for the detection of anti-Borrelia antibodies are revealed by comparing eight ELISAs and five immunoblots. Eur J Clin Microbiol Infect Dis 30:1027–1032
Smit P, Mabey D, Changalucha J et al (2013) The trade-off between accuracy and accessibility of syphilis screening assays. PLoS One 8:e75327
Mygland A, Ljøstad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I (2010) EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol 17(8–16):e1–e4
Mabey D, Peeling RW, Ustianowski A, Perkins MD (2004) Diagnostics for the developing world. Nat Rev Microbiol 2:231–240
Mabey DC, Sollis KA, Kelly HA et al (2012) Point-of-care tests to strengthen health systems and save newborn lives: the case of syphilis. PLoS Med 9:e1001233
Hurly DS, Buhrer-Skinner M, Badman SG et al (2013) Field evaluation of the CRT and ACON chlamydia point-of-care tests in a tropical, low-resource setting. Sex Transm Infect 90:179–184
Herring AJ, Ballard RC, Pope V et al (2006) A multi-centre evaluation of nine rapid, point-of-care syphilis tests using archived sera. Sex Transm Infect 82 [Suppl 5]:v7–v12
Acknowledgements
We would like to thank Aftab Jasir, Androulla Efstratiou, Triin Pärn and Jaana Vuopio for critically proofreading the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smit, P.W., Kurkela, S., Kuusi, M. et al. Evaluation of two commercially available rapid diagnostic tests for Lyme borreliosis. Eur J Clin Microbiol Infect Dis 34, 109–113 (2015). https://doi.org/10.1007/s10096-014-2217-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2217-5