Abstract
We evaluated blood and fecal biomarkers as indicators of severity in symptomatic patients with confirmed Clostridium difficile infection (CDI). Recruitment included patients with CDI based on clinical symptoms and supporting laboratory findings. Disease severity was defined by physician’s assessment and blood and fecal biomarkers were measured. Toxigenic culture done using spore enrichment and toxin B detected by tissue culture were done as confirmatory tests. Polymerase chain reaction (PCR) ribotyping was performed on each isolate. There were 98 patients recruited, with 85 (87 %) confirmed cases of toxigenic CDI (21 severe, 57 moderate, and seven mild), of which 68 (80 %) were also stool toxin-positive. Elevated lactoferrin (p = 0.01), increased white blood cell (WBC) count (p = 0.08), and low serum albumin (p = 0.03) were all associated with the more severe cases of CDI. Ribotype 027 infection accounted for 71 % of severe cases (p < 0.01) and patients with stool toxin had significantly higher lactoferrin levels and WBC counts (p < 0.05). Our findings show that elevated fecal lactoferrin, along with increased WBC count and low serum albumin, were associated with more severe CDI. In addition, patients infected with ribotype 027 and those with stool toxin had significantly higher fecal lactoferrin and WBC counts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
The incidence of Clostridium difficile infection (CDI) is increasing and estimates are now around 500,000 cases per year in the U.S., with a yearly cost of 3.2 billion dollars [2, 8, 23, 24]. An epidemic strain, ribotype 027, has been implicated in a number of outbreaks in Europe, Canada, and the U.S. [2, 6, 10, 12, 13, 36]. This strain has toxin A and toxin B genes (tcdA and tcdB, respectively), genes for a binary toxin, 18-base-pair tcdC deletion (down regulator for the expression of toxin), and fluoroquinolone resistance. Studies have shown that the ribotype 027 strain expresses increased levels of toxin in vitro, more spores, and is associated with severe CDI [10, 21, 22, 26].
Identifying actual cases of CDI and determining disease severity are important factors when recommending a proper course of treatment, including choosing the antibiotic therapy; metronidazole for mild CDI and vancomycin for severe CDI [5, 15, 18, 40]. In general, patients with C. difficile disease often present with fever, have slightly raised white blood cell (WBC) counts (leukocytosis), and experience mild to moderate abdominal pain. Most cases of CDI require discontinuation of inciting antibiotic therapy when possible and the initiation of C. difficile-specific antimicrobial therapy [5, 18]. Defined laboratory parameters for fulminant C. difficile colitis are WBC count ≥15,000/μL, a rising serum creatinine (50 % increase and levels ≥2.0 mg/dL), and albumin levels dropping below 2.5 mg/dL. Clinical complications may involve pseudomembrane formation, severe abdominal pain and/or cramping, and colonic thickening observed by computed tomography (CT) scan. Toxic megacolon stemming from the ileus may occur, causing nausea, vomiting, severe dehydration, and extreme lethargy [5, 7, 12, 13, 35]. Currently, no single laboratory parameter or combination of clinical symptoms is routinely used to stratify patients based on the severity of CDI for directing therapy.
Intestinal inflammation is a hallmark of patients with CDI [13, 35]. The severe stage of CDI, pseudomembranous colitis (PMC), results from the excessive tissue damage and inflammation. Biomarkers that help determine the “inflammatory status” in the intestine of a CDI patient potentially help predict the severity of CDI and possibly help direct the choice of antibiotic therapy [16]. Fecal lactoferrin is a biomarker for intestinal inflammation and has been evaluated in both infectious diarrhea and inflammatory bowel disease [9, 11, 16, 30–32, 34, 38]. Previous studies have demonstrated that elevated lactoferrin occurs in patients with C. difficile disease [1, 17, 25, 39]. Although these studies identified increased fecal lactoferrin in patients with CDI, none correlated the significance of levels detected in patients with defined CDI characteristics in combination with blood biomarkers for assessing disease severity. Defining a diagnostic role for blood and fecal biomarkers of inflammation for stratifying CDI patients according to disease severity would offer a clinical index for optimizing treatment and improving patient outcomes.
In this study, we evaluated blood and fecal biomarkers as indicators of severity in symptomatic patients with confirmed CDI by toxigenic culture. Patients were stratified by disease severity according to a physician’s assessment, presence of ribotype 027 infection, and the detection of stool toxin.
Materials and methods
Study design and fecal specimens
The study protocol and recruiting method was approved by the institutional review boards (IRBs) of the Summa Health System (Akron, Ohio) and TechLab, Inc. (Blacksburg, Virginia). Recruitment was done between February 2010 and September 2012 using informed consent and included adult hospitalized patients and outpatients that were identified as having CDI based on a combination of symptoms, including ≥3 stools per day and in vitro diagnostic testing for glutamate dehydrogenase (GDH) and stool toxin performed by the clinical microbiology laboratory at Summa Health System. Patients with documented enteric infection other than C. difficile, inflammatory bowel disease, irritable bowel syndrome, and celiac disease were excluded. Each patient provided a single stool specimen for clinical diagnosis and, if CDI-positive, a portion of this specimen was then shipped to TechLab, Inc. for tissue culture testing, toxigenic culture, ribotyping of isolates, and fecal lactoferrin analysis. Stool testing was done within 72 h of collection by the clinical laboratory or after a single freeze–thaw at TechLab. Patient information and stool specimens were coded to maintain anonymity. Specimen consistency was defined according to the Bristol Stool Chart as follows: liquid specimens were defined as type 7; partially formed and soft specimens were defined as types 4–6; formed specimens were defined as types 1–3 [19, 22].
Chart reviews and disease assessment
Patient chart reviews were performed to record patient age, gender, co-morbidities, antibiotic use, treatment outcomes, reported symptoms, blood analysis for WBC count, and serum albumin level. Disease severity for CDI was done by physician’s assessment using the following guidelines: (i) mild CDI cases included patients of any age, but usually ≤65 years, WBC <15 × 109/L, stool <5 per day, no abdominal, peritoneal, or radiographic signs of disease, no co-morbid conditions, and able to tolerate oral intake; (ii) moderate CDI cases were of any age, WBC <15 × 109/L, stool ≥5 per day, usually able to tolerate oral intake, reported abdominal complaints, had radiographic or peritoneal signs, existing co-morbid conditions including but not limited to renal failure and immunosuppression; (iii) severe CDI cases involved patients of any age, but was automatically classified as severe if age ≥65 years, WBC >15 × 109/L, stool ≥10 per day, not able to tolerate oral intake, usually abdominal complaints, radiographic or peritoneal signs, multiple co-morbidities including but not limited to renal failure and immunosuppression.
Tissue culture for toxin B
The C. DIFFICILE TOX-B Test (TechLab® Inc., Blacksburg, VA) was performed according to the manufacturer’s package insert with modification to include a neutral goat serum control. Briefly, a total of 50 μl of fecal supernatant was added to each of three wells containing a confluent layer of human foreskin cells and 50 μl of one of the following: PBS, C. difficile antitoxin, and neutral goat serum. A positive cytotoxic effect was defined as greater than 50 % rounding in the wells containing neutral goat serum and PBS but neutralized with no rounding in wells containing antitoxin.
Qualitative detection of GDH and toxin by immunoassay
The C.DIFF QUIK CHEK COMPLETE™ membrane-based enzyme-linked immunosorbent assay (ELISA) test (TechLab® Inc., Blacksburg, VA) was performed according to the manufacturer’s package insert.
Quantitative fecal lactoferrin
Fecal lactoferrin concentrations were determined by immunoassay using the IBD-SCAN® test (TechLab®, Inc., Blacksburg, VA). A clinical cut-off of ≥7.25 μg/g stool was used to define an elevated level of fecal lactoferrin as an indicator of intestinal inflammation [11].
Toxigenic culture
The isolation of C. difficile was done using the method previously described by Boone et al. [4].
PCR analysis and ribotyping
C. difficile isolates were polymerase chain reaction (PCR) ribotyped using the procedure developed by Stubbs et al. [33].
Statistical analysis
Normal distribution was confirmed prior to analysis. Median, standard deviation, standard error, and significance were generated using the JMP 9 (Cary, NC) statistical program by analysis of variance (ANOVA), Student’s t-test, or the Chi-square likelihood ratio test, depending upon the data type examined. Student’s t- test was used when comparing two independent sets of continuous data with assumed equal variances. Chi-square was employed to determine the significance between two groups of categorical data within a contingency table of a large sample size having only one degree of freedom. The clinical significance of severe CDI was determined using one-way ANOVA and expressed as p-values. A multilinear regression model that included combined patient variables was used to investigate predictors of severe CDI. For this study, the mean results were reported with standard error (SE) and significance was defined as having a p < 0.05. The range of levels for each biomarker is provided as a minimum and maximum (min, max).
Results
A total of 98 patients identified as having CDI based on a combination of symptoms, history of antibiotic use, stool GDH, and toxin were enrolled into the study. The mean age was 67 years and 64 % were female (Table 1). There were 85 (87 %) confirmed cases of toxigenic CDI using toxigenic culture, of which 68 (80 %) had stool toxin detected by the tissue culture assay. Of these, 38 (45 %) were infected with ribotype 027. One patient had a mixed infection of toxigenic and nontoxigenic C. difficile based on the isolation of a nontoxigenic ribotype (009) in combination with the detection of stool toxin by the tissue culture assay. This patient was admitted directly from an extended-care facility and had previous antibiotic therapy. Fecal lactoferrin (15 μg/g) and WBC count (11.9 × 109/L) were slightly elevated and the serum albumin level was low (2.4 mg/dL). Repeat bacterial culture never produced a toxigenic isolate. Six patients (6 %) were negative for stool toxin and colonized with nontoxigenic isolates, as demonstrated by the isolation of nontoxigenic ribotypes, including 009 (1), 010 (1), 038 (2), and 039 (2). Repeated attempts to isolate a toxigenic ribotype were unsuccessful. The remaining six patients were negative for C. difficile by both bacterial culture and tissue culture. Additional patient characteristics including reported symptoms, co-morbidity, and treatment history are listed in Table 1.
Patients infected with toxigenic C. difficile were stratified according to physician’s assessment for disease severity, resulting in 21 severe cases, 57 moderate cases, and seven mild cases. Age, reported abdominal pain, number of stools per day, blood, and fecal biomarkers were evaluated between severity groups and the results are shown in Table 2. Patients who suffered with clinically severe CDI had significantly elevated lactoferrin, higher WBC counts, and lower serum albumin when compared to those cases with moderate and mild clinical disease. In addition, we evaluated the clinical impact for blood and fecal biomarkers with severe CDI using one-way ANOVA. We identified a significant impact (p < 0.05) for increased lactoferrin, high WBC count, low serum albumin, and ribotype 027 infection for severe CDI (Table 2). In addition, we utilized a multilinear regression model that included age, presence of pain, stool toxin, lactoferrin, serum albumin, WBC count, and stools per day to investigate predictors of severe CDI. The model showed a significant association between these variables and severe CDI (p = 0.0175). The effect weight for each variable in the model expressed as a p-value is shown Table 3. Of the biomarkers included in the model, lactoferrin and serum albumin levels were highly significant (p < 0.05) and the WBC count trended close to being significant (p = 0.0802).
In our population, we had an overall 027 infection rate of 45 %, with the next four most common non-027 toxigenic ribotypes being 014 (8 %), 106 (5 %), 002 (5 %), and 126 (4 %). There were six non-027 isolates that were nontoxigenic and these patients were not included in the analysis for evaluating disease severity. There was a significant difference for elevated lactoferrin (p = 0.012) and for lower serum albumin levels (p = 0.003) between 027-infected patients compared to other toxigenic non-027-infected patients. Age, WBC count, number of stools per day, and reported abdominal pain were similar between the ribotype groups.
Testing for stool toxin by tissue culture showed that 80 % of patients infected with a toxigenic ribotype had detectable toxin. When patients were stratified based on the presence of stool toxin, toxin-positive patients had significantly higher lactoferrin (p = 0.019) and WBC count (p = 0.029), and the serum albumin levels trended lower (Table 4). The number of toxin-positive patients reporting abdominal pain trended towards significance (p = 0.054) and there was a significantly higher number of toxin-positive patients with ribotype 027 infection (p = 0.044). There were a total of 19 patients that required additional antibiotics for their CDI episode and 90 % of these patients had stool toxin and 68 % of them were infected with ribotype 027. For this group, the mean ± SE for lactoferrin, WBC count, and serum albumin was 566 ± 261, 17.1 ± 3.8, and 2.3 ± 0.2, respectively. No difference was observed between groups for age and the number of stools per day. When stratified by gender, male patients were significantly more likely to be stool toxin-positive compared to female patients (p = 0.002). Of the patients with detectable stool toxin, the levels of lactoferrin ranged from 1 to 4,672 μg/g feces, and only 3 (4 %) patients had normal fecal lactoferrin levels (<7.25 μg/g feces). For patients that were negative for stool toxin, 41 % had normal lactoferrin and all but a single patient had levels below 85 μg/g feces.
Discussion
In this study, we examined blood and fecal biomarkers for assessing disease severity in patients with CDI. We measured two commonly used blood parameters for assessing disease severity in CDI, WBC count and serum albumin level, for comparison with fecal lactoferrin levels as an indicator of intestinal inflammation. C. difficile-specific diagnostic biomarkers including GDH, toxin B, and ribotype 027 isolates were also measured. Our results showed significantly more inflammation as determined by increased WBC counts (peripheral) and elevated fecal lactoferrin (intestinal) for patients with clinically assessed moderate to severe CDI. Serum albumin was lower in patients with more severe disease, indicating a damaged mucosa, resulting in the loss of serum protein into the bowel. A multilinear regression model examining the relationship of clinical symptoms and biomarkers on disease severity indicated a strong association for severe CDI (p = 0.0175). In addition, the fecal lactoferrin and blood biomarkers, WBC count and low serum albumin level showed a significant association within severe CDI (p < 0.05). Further studies are needed for evaluating the combination of fecal lactoferrin and blood biomarkers with symptoms and patient characteristics for developing a sensitive and specific disease severity index for stratifying CDI patients for optimal treatment and disease management.
Another important finding of our study is the clinical importance of determining stool toxin as an indicator of disease. Our results showed that elevated biomarkers of inflammation, WBC count and lactoferrin, were associated with the presence of stool toxin. Patients with clinically assessed severe and moderate CDI were more often likely to have a positive stool toxin (80 %). The combination of stool toxin and increased intestinal inflammation as shown by increased lactoferrin is not an unexpected finding because: (i) both toxins A and B are strong chemotactic proteins that cause the release of IL-8 and the infiltration of activated neutrophils into the intestinal mucosa [29]; (ii) toxin A also stimulates other pro-inflammatory cytokines, including IL-1β and tumor necrosis factor alpha (TNF), while toxin B is a cytotoxin that causes both tissue damage and inflammation [14, 20, 28, 29]; and (iii) the combined effects of the tissue damaging and chemotactic activities of toxins A and B contribute to the severity of CDI [29]. Recently, Planche et al. analyzed 12,420 specimens from 10,691 patients using the reference assays toxigenic culture and tissue culture assay for stool toxin. Patients with stool toxin had a significantly higher mortality rate (16.6 %) compared to the toxigenic culture-positive only (9.7 %; p = 0.022) and C. difficile-negative groups (8.6 %; p = 0.001). In addition, the stool toxin-positive patients had significantly higher (p = 0.001) WBC counts compared to the other two groups. Based on these results, Planche et al. concluded that patients having a positive stool toxin have C. difficile disease with an increased risk of mortality [27]. Considering our findings along with the Planche et al. study, a method for determining the presence of intestinal inflammation in combination with the presence of toxin in stool identifies patients with severe CDI. In addition, even though quantifying the amount of toxin in the stool was beyond the initial scope of this study, future work should consider measuring the level of toxins A and B and the correlation of amounts to biomarkers of inflammation and severity of disease.
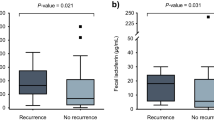
A new finding from this study is the correlation of increasing fecal lactoferrin levels with increasing severity of clinically assessed CDI. Lactoferrin levels showed over a 5-fold increase in patients classified as severe CDI compared to moderate disease. Multiple studies have demonstrated increased WBC count as a useful indicator of severity in CDI; however, it is worth noting that these counts are not specific for intestinal inflammation and may be increased by inflammatory co-morbidities. Fecal lactoferrin is a glycoprotein that is present in most mucosal secretions and a primary component of the granules of activated neutrophils; thus, it is a specific marker of intestinal inflammation. During the onset of intestinal inflammation caused by C. difficile, activated neutrophils infiltrate the intestinal lumen, causing an increase in fecal lactoferrin [9, 11]. Our results support a role for assessing patients with CDI using fecal lactoferrin as an indicator of intestinal inflammation and then using the amount as an aid for stratifying patients based on disease severity. In addition, a biomarker like fecal lactoferrin may offer the utility for monitoring disease activity in patients with CDI as an indicator of response to medical treatment and for predicting a relapse.
The emergence of ribotype 027, which has been shown to express in vitro, more toxin, and production of increased spores has been linked to numerous outbreaks involving more severe C. difficile disease and a greater chance of relapse compared to other known ribotypes [10, 12, 21, 22, 26]. However, some recent studies have shown no link between the infecting ribotype and more severe clinical disease and worse outcomes, raising questions on the clinical significance, in terms of severity, of an 027 infection [3, 23, 37]. In our study population, we had 45 % of patients with toxigenic C. difficile infected with ribotype 027. These patients were more likely to be assessed as severe by the physician and had significantly higher lactoferrin indicating more intestinal inflammation, lower serum albumin levels, and a higher frequency of positive stool toxin (92 %). More research is needed in order to determine the in vivo production of increased stool toxin in patients infected with 027 as a virulence factor causing more inflammation, resulting in severe CDI.
In conclusion, our results show increased fecal lactoferrin, higher WBC counts, and lower serum albumin levels in CDI patients who have severe clinical disease. The presence of stool toxin was associated with increased inflammation for moderate and severe cases. In addition, we confirmed that older, sicker patients with ribotype 027 CDI are more likely to be stool toxin-positive, resulting in increased intestinal and peripheral inflammation, as indicated by higher levels of fecal lactoferrin and WBC counts, respectively. Future studies are needed in order to evaluate the optimization of treatment based on a clinical index for severity that combines patient characteristics with fecal and blood biomarkers in patients infected with C. difficile.
References
Archbald-Pannone L, Sevilleja JE, Guerrant R (2010) Diarrhea, Clostridium difficile, and intestinal inflammation in residents of a long-term care facility. J Am Med Dir Assoc 11(4):263–267
Bartlett JG, Perl TM (2005) The new Clostridium difficile—what does it mean? N Engl J Med 353:2503–2505
Barbut F, Rupnik M (2012) Editorial commentary: 027, 078, and others: going beyond the numbers (and away from the hypervirulence). Clin Infect Dis 55(12):1669–1672
Boone JH, Goodykoontz M, Rhodes SJ, Price K, Smith J, Gearhart KN et al (2012) Clostridium difficile prevalence rates in a large healthcare system stratified according to patient population, age, gender, and specimen consistency. Eur J Clin Microbiol Infect Dis 31(7):1551–1559
Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC et al (2010) Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 31(5):431–455
Drudy D, Kyne L, O’Mahony R, Fanning S (2007) gyrA mutations in fluoroquinolone-resistant Clostridium difficile PCR-027. Emerg Infect Dis 13:504–505
Eyre DW, Walker AS, Wyllie D, Dingle KE, Griffiths D, Finney J, et al (2012) Predictors of first recurrence of Clostridium difficile infection: implications for initial management. Clin Infect Dis 55:S77–S87
Ghantoji SS, Sail K, Lairson DR, Dupont HL, Garey KW (2010) Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect 74:309–318
Guerrant RL, Araujo V, Soares E, Kotloff K, Lima AA, Cooper WH et al (1992) Measurement of fecal lactoferrin as a marker of fecal leukocytes. J Clin Microbiol 30:1238–1242
Hubert B, Loo VG, Bourgault AM, Poirier L, Dascal A, Fortin E et al (2007) A portrait of the geographic dissemination of the Clostridium difficile North American pulsed-field type 1 strain and the epidemiology of C. difficile-associated disease in Québec. Clin Infect Dis 44:238–244
Kane SV, Sandborn WJ, Rufo PA, Zholudev A, Boone J, Lyerly D et al (2003) Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol 98(6):1309–1314
Kazakova SV, Ware K, Baughman B, Bilukha O, Paradis A, Sears S et al (2006) A hospital outbreak of diarrhea due to an emerging epidemic strain of Clostridium difficile. Arch Intern Med 166:2518–2524
Kelly CP, Loo VG (eds) (2008) Current concepts in Clostridium difficile infection: a focus on severe disease. CME Newsl vol 2, issue 2, pp 1–6
Kim H, Rhee SH, Pothoulakis C, LaMont JT (2007) Inflammation and apoptosis in Clostridium difficile enteritis is mediated by PGE2 up-regulation of Fas ligand. Gastroenterology 133:875–886
Koo HL, Ajami NJ, Jiang ZD, Dupont HL, Atmar RL, Lewis D et al (2009) A nosocomial outbreak of norovirus infection masquerading as Clostridium difficile infection. Clin Infect Dis 48:e75–e77
Langhorst J, Boone J (2012) Fecal lactoferrin as a noninvasive biomarker in inflammatory bowel diseases. Drugs Today (Barc) 48(2):149–161
LaSala PR, Ekhmimi T, Hill AK, Farooqi I, Perrotta PL (2013) Quantitative fecal lactoferrin in toxin-positive and toxin-negative Clostridium difficile specimens. J Clin Microbiol 51(1):311–313
Leffler DA, Lamont JT (2009) Treatment of Clostridium difficile-associated disease. Gastroenterology 136:1899–1912
Lewis SJ, Heaton KW (1997) Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32(9):920–924
Linevsky JK, Pothoulakis C, Keates S, Warny M, Keates AC, Lamont JT et al (1997) IL-8 release and neutrophil activation by Clostridium difficile toxin-exposed human monocytes. Am J Physiol 273:G1333–G1340
Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S et al (2005) A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 352:2442–2449
McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, Sambol SP et al (2005) An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 353:2433–2441
Morgan OW, Rodrigues B, Elston T, Verlander NQ, Brown DF, Brazier J et al (2008) Clinical severity of Clostridium difficile PCR ribotype 027: a case–case study. PLoS One 3:e1812
O’Brien JA, Lahue BJ, Caro JJ, Davidson DM (2007) The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol 28:1219–1227
Pawlowski SW, Archbald-Pannone L, Carman RJ, Alcantara-Warren C, Lyerly D, Genheimer CW et al (2009) Elevated levels of intestinal inflammation in Clostridium difficile infection associated with fluoroquinolone-resistant C. difficile. J Hosp Infect 73(2):185–187
Pépin J, Valiquette L, Cossette B (2005) Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 173(9):1037–1042. doi:10.1503/cmaj.050978
Planche TD, Davies KA, Coen PG, Crook D, Shetty N, Wren M et al (2012) Clinical validation of Clostridium difficile infection (CDI) diagnostics: importance of toxin detection. In: Proceedings of the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, CA, September 2012
Pothoulakis C, Sullivan R, Melnick DA, Triadafilopoulos G, Gadenne AS, Meshulam T et al (1988) Clostridium difficile toxin A stimulates intracellular calcium release and chemotactic response in human granulocytes. J Clin Invest 81:1741–1745
Savidge TC, Pan WH, Newman P, O’Brien M, Anton PM, Pothoulakis C (2003) Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 125:413–420
Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F (2008) Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis 14:32–39
Sipponen T, Savilahti E, Kärkkäinen P, Kolho KL, Nuutinen H, Turunen U et al (2008) Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn’s disease. Inflamm Bowel Dis 14(10):1392–1398
Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M (2007) Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis 14:40–46
Stubbs SL, Brazier JS, O’Neill GL, Duerden BI (1999) PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J Clin Microbiol 37:461–463
Sugi K, Saitoh O, Hirata I, Katsu K (1996) Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am J Gastroenterol 91(5):927–934
Surawicz CM, McFarland LV (1999) Pseudomembranous colitis: causes and cures. Digestion 60:91–100
Tan ET, Robertson CA, Brynildsen S, Bresnitz E, Tan C, McDonald C (2007) Clostridium difficile-associated disease in New Jersey hospitals, 2000–2004. Emerg Infect Dis 13:498–500
Walk ST, Micic D, Jain R, Lo ES, Trivedi I, Liu EW et al (2012) Clostridium difficile ribotype does not predict severe infection. Clin Infect Dis 55(12):1661–1668
Walker TR, Land ML, Kartashov A, Saslowsky TM, Lyerly DM, Boone JH et al (2007) Fecal lactoferrin is a sensitive and specific marker of disease activity in children and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 44:414–422
Wren MW, Kinson R, Sivapalan M, Shemko M, Shetty NR (2009) Detection of Clostridium difficile infection: a suggested laboratory diagnostic algorithm. Br J Biomed Sci 66(4):175–179
Zar FA, Bakkanagari SR, Moorthi KM, Davis MB (2007) A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 45:302–307
Acknowledgments
Funding for this study was provided by TechLab, Inc.
Conflict of interest
The authors include scientists of TechLab, Inc. Dr. Michael Tan is a member of the Speaker Bureau for Optimer Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Boone, J.H., DiPersio, J.R., Tan, M.J. et al. Elevated lactoferrin is associated with moderate to severe Clostridium difficile disease, stool toxin, and 027 infection. Eur J Clin Microbiol Infect Dis 32, 1517–1523 (2013). https://doi.org/10.1007/s10096-013-1905-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-013-1905-x