Abstract
The aim of this study was to survey the occurrence of invasive group B streptococcus (GBS) disease in Norway and detect possible trends in characteristics of invasive GBS strains from1996 to 2006. Data from national monitoring systems for infectious diseases in Norway were analysed. Of 638,452 live births in the period, 434 cases of invasive GBS disease in infants were reported. In adults and children older than 1 year of age, 969 cases were reported. The incidence of invasive GBS disease increased significantly in the elderly, while the incidence of neonatal early-onset disease was stable with 0.46 cases per 1,000 live births. The incidence of late-onset disease increased in 2005 and 2006. The lethality of GBS in infants increased from an average of 6.5% in 1996–2005 to 20% in 2006. Serotypes III and V were predominant in 839 invasive GBS strains characterized—type III in infants and type V in the elderly. The distribution of serotypes did not change throughout the period. The distribution of detected surface proteins was stable from 1996 to 2005, but the detection rates in types III and V were low. Molecular methods for GBS typing introduced in 2006 made characterization of nearly all strains possible and appear more applicable to epidemiological studies of GBS than conventional methods. Resistance to erythromycin and clindamycin increased significantly in 2006. The increased incidence in the elderly, the increased lethality in infants in 2006, and the increased resistance to erythromycin and clindamycin the same year might indicate changing characteristics of invasive GBS strains.
Similar content being viewed by others
Introduction
Streptococcus agalactiae (group B streptococcus, GBS) has been an important cause of morbidity and mortality in newborns over the last four decades. The reported incidence of neonatal GBS disease from 1990 to 2005 ranges from <0.5 to 2 per 1,000 live births in different geographic areas [1–3]. Neonatal GBS disease presents as early-onset disease (EOD) or late-onset disease (LOD). In EOD (age at onset 0–6 days), the neonate is infected by exposure to GBS before or during birth. In LOD (age at onset 7–89 days) the pathogenesis is not clear. Intrapartum antibiotics given to women with risk factors for EOD has reduced the incidence of invasive GBS disease in newborns, and different prophylactic strategies to detect women at risk have been recommended [4, 5]. During recent decades, an increasing incidence of invasive GBS disease in adults has been reported worldwide [6–8]. Disease rates increase with age, and disease occurs mainly in those with an underlying medical condition [9].
Almost all clinical isolates of GBS carry a capsular polysaccharide (CPS) and can be classified into nine distinct serotypes or CPS types: Ia, Ib, and II-VIII [10]. In addition, a capsular type IX was proposed in 2007 [11]. Antibodies against CPS provide type-specific protection [12], and conjugate vaccines composed of CPS and tetanus toxoid have been evaluated in healthy adults [13, 14]. The prevalence of serotypes varies with time and geographical location; thus, knowledge of serotype distribution is necessary for the selection and development of serotype-based vaccines in a given geographic area [15, 16]. By combining serotyping with subtyping (testing for expression of strain variable surface proteins or genes that encode the surface proteins), the characterization of GBS can be more precise. Some of the best characterized surface proteins are the c proteins (alpha and beta) and the R proteins (R1, R3, and R4) [17], where R4 is identical to rib [18, 19]. The genes bca, bac, alp1 (epsilon), alp2/alp3, and rib are encoding the proteins alpha c, beta c, alp1 (epsilon), alp2, alp3, and R4 (rib), respectively. A serovariant is the combination of serotype and surface protein, or the gene that encodes the protein. Animal studies have shown that antibodies to the proteins confer protection against GBS [20]. Increasing evidence suggests that surface proteins contribute to virulence and thus will be of relevance for vaccine development [21].
The aims of this study were to survey the incidence of invasive GBS disease in infants and adults and detect possible trends in characteristics of invasive GBS strains in Norway during the period 1996 to 2006.
Patients and methods
Data were obtained from The Norwegian Surveillance system for Communicable Diseases (MSIS) provided by The Norwegian Institute of Public Health (NIPH). MSIS is the official monitoring system for infectious diseases in Norway. Laboratory confirmed cases of invasive GBS disease are compulsorily notifiable in Norway and have been reported to the MSIS database consecutively by all medical microbiological laboratories since 1985. Statistics on population in different age groups, births, and mortality are from Statistics Norway (www.ssb.no).
Characterization of GBS strains
The Department of Medical Microbiology, St. Olavs Hospital, Trondheim is the national reference laboratory for GBS in Norway. The laboratory receives strains from microbiological laboratories for capsular typing, subtyping, and in certain cases genotyping (pulsed-field gel electrophoresis [PFGE] and multilocus sequence typing [MLST]). Unlike the MSIS report system, forwarding strains to the reference laboratory is not compulsory. In the period 1996–2005, GBS strains from 56% of the cases reported to MSIS were characterized. However, in 2006, the reference laboratory received GBS strains from 84% of all cases reported to MSIS and from 100% of the infant cases.
Capsular polysaccharide (CPS) antigen typing was done by an indirect fluorescent antibody test (FAT) [22]. From January 2006, this method was replaced by a PCR method to detect capsular polysaccharide synthesis gene clusters [23, 24].
From 1996–2005 antibody-based detection of surface proteins (sero-subtyping) was performed using murine monoclonal antibodies against alpha c protein, beta c protein, and R4 in an indirect whole cell-based fluorescent antibody test (FAT) [25]. From January 2006, this method was replaced by molecular methods detecting the genes bca, bac, alp1 (epsilon), alp2/alp3, and rib [26, 27]. The genes alp2 and alp3 were not tested for separately.
Since 2003, all GBS isolates were tested for susceptibility to erythromycin and clindamycin by agar diffusion and analysed according to interpretational criteria recommended by the Norwegian AFA Group (www.antibiotikaresistens.no). Erythromycin resistant, clindamycin sensitive strains were tested with erythromycin and clindamycin tabs or discs, with inner edges 25 mm apart. Strains with D-shaped clindamycin zones (inducible clindamycin resistance) were classified as resistant to clindamycin (www.srga.org).
Statistics
Data were collected and analysed using Windows Office Excel and SPSS software (SPSS Inc., Chicago, IL). Minitab and Pearson’s chi-square test were used for comparison of proportions of serotypes and incidences of invasive GBS disease.
Results
There were 4,681,100 inhabitants in Norway as of January 1, 2007. From 1996 to 2006, there were 1,403 cases of invasive GBS disease in infants, children, and adults reported to MSIS.
GBS disease in infants
From 1996 to 2006 there were 638,452 live births (range, 55,434–60,927 per year) in Norway. In this period 422 infants with GBS disease younger than 90 days old were reported to MSIS. The cumulative incidence of infants <90 days old was 0.66 per 1,000 live births (annual range, 0.38–1.0/1000), and the cumulative incidence of EOD was 0.46 per 1,000 live births (range, 0.28–0.83/1000). The incidence of invasive GBS in infants showed no significant trend throughout the study period (p = 0.2). However, the proportion of LOD increased during the last two years of the period of observation, from 28% (range, 16.9–33.3%) of invasive disease in 1996–2004 to 42.7% (42.5–42.9%) in 2005 and 2006 (p = 0.006). Meningitis was reported in 25 infants (6%). Reported case fatality of newborns and infants with GBS disease was 6.5% (range, 1–5 deaths per year) from 1996–2005. In 2006 the case fatality increased to 20% (p = 0.02) with ten deaths caused by GBS.
GBS disease in adults and children >1 year old
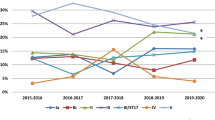
During the period 1996–2006, 969 cases of invasive GBS disease in adults and children >1 year were reported to MSIS. The annual numbers increased during the period. The increasing incidence was most obvious in the elderly >70 years since 1999, and from 2004 also in the age groups 50–69 years (Fig. 1). The overall mean incidence of GBS disease in adults (>19 years) increased from 1.34 cases per 100,000 in 1996–1998 to 3.1 cases per 100,000 in 1999–2006 (p < 0.001). The mean incidence in the elderly (>70 years) increased from 3.9 per 100,000 in 1996–1998 to 9.15 in 1999–2006 (p < 0.001) (Fig. 1).
Characterization of invasive GBS strains
From 1996 to 2006 capsular type III was predominant in strains from newborns and infants, while capsular type V was predominant in the elderly (Fig. 2). The other capsular types were evenly distributed in different age groups (Fig. 2). There was no significant change in the frequency of any of the serotypes throughout the period (results not shown). The most common surface proteins observed were rib (30% in infants and 15.1% in adults) and alpha c (20.2% in infants and 25.2% in adults) (Table 1). The distribution of surface proteins and serovariants did not change significantly in the period from 1996 to 2005 (results not shown).
From infants <1 year the reference laboratory received 218 GBS strains (58% of MSIS reported cases) from cases of invasive disease during the period 1996–2005. Of these 218 strains, 141 were from neonates with EOD and 68 from LOD. In addition, nine strains were from infants <1 year, but without information of onset of the disease. Most of these strains (n = 209) were isolated from blood cultures—eight from CSF and one from synovia. Serotype III was the most common serotype (53%) followed by serotype Ia (12.8%), V (11.9%), and Ib (10.1%) (Table 1). Serotype III was more frequent in LOD than in EOD (63.2% vs. 48.2%, respectively) (p = 0.04) (Fig. 2). Seven of eight strains recovered from cerebrospinal fluid were serotype III. Surface proteins were detected in the majority of type Ia, Ib, II, and IV strains, fewer in type III (48%), and in only 27% of type V (Table 1).
In 2006, invasive strains from 55 infants (110% of MSIS reported cases) were received and analyzed by the reference laboratory, including 47 strains from blood cultures and eight from CSF. The distribution of serotypes was similar to the period from 1996 to 2005 (Table 2). Genes encoding surface proteins were detected in all but two strains (Table 2).
From adults and children >1 year, isolates recovered from 465 cases of invasive GBS (55% of MSIS reported cases) were analyzed in the period 1996–2005. Serotypes III (24.5%) and V (24.1%) were the most frequent serotypes (Table 1). The rank order of serotype frequency among people >70 years did not change significantly after 1998 when the incidence of GBS increased; neither did it change in the age group 50–69 years after 2003 when a similar increase in incidence was observed (Fig. 1). Surface proteins were detected in the majority of types Ia, Ib, II, and IV strains, fewer in type III (40.3%), and 19% of type V (Table 1).
In 2006, 101 invasive GBS strains from adults and children >1 year (78% of MSIS reported cases) were analyzed. Serotypes were distributed similarly as in the period from 1996 to 2005 (Table 2). Genes for surface proteins were detected in 97 of 101 strains (Table 2).
Resistance to clindamycin and erythromycin
From 2003 to 2005, 4% of 75 strains from infants were resistant to clindamycin and erythromycin. In adults, 1.4% of 208 isolates were resistant to clindamycin, and 3.4% were resistant to erythromycin in the same period. Only one case of inducible clindamycin resistance was observed. In 2006, however, 14 of 55 strains (25.4%) from infants were resistant to erythromycin and clindamycin, of which ten showed inducible clindamycin resistance. Seven of ten strains with inducible clindamycin resistance in infants were serotype V of which five were identical or closely related as analysed by pulsed-field gel electrophoresis (PFGE) (result not shown). The last three strains with inducible clindamycin resistance were serotype III. Inducible clindamycin resistance was observed in four of the ten strains from fatal cases in infants in 2006, all of which were all type V. In 2006, 11.9% of 101 strains from adults were resistant to erythromycin and 10.9% to clindamycin. Nine of the twelve resistant strains showed inducible clindamycin resistance.
Discussion
The incidence of EOD in Norway was 0.3 per 1,000 in the period 1986 to 1992, but increased to 0.47 per 1,000 between 1992 and 1994 [28]. After 1994, the incidence of GBS disease in newborns and infants has remained unchanged. The mean incidence of EOD in 1996–2006 (0.46/1000) was lower than reported in Finland (0.62/1000) but higher than reported in Germany (0.28/1000) and the USA (0.37/1000) [3, 29, 30]. However, the incidence of GBS disease in newborns and infants is probably underestimated worldwide due to a significant number of culture-negative sepsis-like syndromes in neonates. Studies have estimated a total incidence of EOD three to four times higher than the incidence of confirmed disease [31]. Therefore, it is difficult to interpret and compare incidences between countries and from one period to another.
The increasing rates of GBS disease in adults may be attributed in part to an expanding population of elderly who are living longer with significant underlying medical conditions. However, this does not explain the apparent rapid change occurring in Norway after 1998. There has been no change of diagnostic methods or routines for surveillance in recent decades. A higher prevalence of more virulent GBS strains in the population might explain the increased incidence.
In general, the serotype distribution was similar to what has been found in other countries [15, 32, 33]. Serotype V strains with identical or similar PFGE patterns were found in four of the ten deaths in infants in 2006 and were also predominant among the observed resistant GBS strains the same year. This may indicate circulation or introduction of a more virulent GBS type V strain in Norway. However, these findings were based on small numbers and in 2006 only. Thus, further observation of incidences, lethality, and characterization of invasive strains is required in the future.
Identification of certain surface proteins or genes encoding surface proteins in invasive GBS strains from Norway might have indicated candidate components of a GBS vaccine. However, no single surface protein or gene was sufficiently common to fulfil this purpose, and further, the detection rate of surface proteins in type III and V strains was low. The low detection rate of surface proteins in type V is probably due to the fact that type V often carries R1 surface protein [34] which was not tested for in this study. In type III strains with no detected surface proteins, the cause might be expression of no or very low levels of R4 proteins as shown by Maeland et al. [34]. Genes encoding surface proteins were detected in nearly all strains (Table 2). All genes (Table 2), except alp1(epsilon) which was more common in strains from adults in Norway in 2006, were similarly distributed as in strains from western Sweden [35].
The change of typing method as of January 1, 2006 was an administrative decision and not influenced by the later observed increased lethality in infants, but might represent a bias in our study. However, we consider the distribution of serotypes in 2006 comparable to the period from 1996 to 2005 as former studies have shown a good correlation between conventional serotyping and typing with molecular methods [23, 36]. Antibody-based methods for serotyping and subtyping require large laboratory resources. In addition, the molecular methods for typing of GBS strains provide a better typability than conventional methods and are therefore more suitable for epidemiological studies of GBS.
In the period 1996–2005 the reference laboratory characterized strains from 56% of MSIS reported cases while in 2006 the reference laboratory characterized strains from all MSIS reported cases in infants and 78% of MSIS reported cases in adults. This increase was due to a decree issued by the health authorities in 2006, requesting medical microbiological laboratories to forward invasive GBS strains to the reference laboratory. Although this increased number and proportion of strains received in 2006 might represent a bias in our material, the characterization of a nearly complete nationwide collection of invasive GBS strains gives a more comprehensive picture of GBS in Norway.
In conclusion, the overall incidence of invasive GBS disease in Norway increased significantly from 1996 to 2006. This was entirely due to an increase among the elderly.
After 1998 more than two thirds of all invasive GBS disease in Norway occurred in adults. The data demonstrate a stable incidence of early-onset GBS disease in Norway (0.46/1000 live births), but the increased incidence in the elderly, the increased lethality in infants in 2006, and the increased resistance to erythromycin and clindamycin the same year, might indicate changing characteristics of GBS strains. Although based on small numbers, these indications of change underline the need for continuous surveillance.
References
Trijbels-Smeulders MA, Kollee LA, Adriaanse AH, Kimpen JL, Gerards LJ (2004) Neonatal group B streptococcal infection: incidence and strategies for prevention in Europe. Pediatr Infect Dis J 23:172–173. DOI 10.1097/01.inf.0000111212.94913.5f
Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, Hadler JL, Danila R, Cieslak PR, Schuchat A (2000) Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 342:15–20. DOI 10.1056/NEJM200001063420103
Lyytikainen O, Nuorti JP, Halmesmaki E, Carlson P, Uotila J, Vuento R, Ranta T, Sarkkinen H, Ammala M, Kostiala A, Jarvenpaa AL (2003) Invasive group B streptococcal infections in Finland: a population-based study. Emerg Infect Dis 9:469–473
Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A (2002) Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 51:1–22
Boyer KM, Gotoff SP (1986) Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med 314:1665–1669
Munoz P, Llancaqueo A, Rodriguez-Creixems M, Pelaez T, Martin L, Bouza E (1997) Group B streptococcus bacteremia in nonpregnant adults. Arch Intern Med 157:213–216. DOI 10.1001/archinte.157.2.213
Tyrrell GJ, Senzilet LD, Spika JS, Kertesz DA, Alagaratnam M, Lovgren M, Talbot JA (2000) Invasive disease due to group B streptococcal infection in adults: results from a Canadian, population-based, active laboratory surveillance study–1996. Sentinel Health Unit Surveillance System Site Coordinators. J Infect Dis 182:168–173. DOI 10.1086/315699
Edwards MS, Baker CJ (2005) Group B streptococcal infections in elderly adults. Clin Infect Dis 41:839–847. DOI 10.1086/432804
Farley MM (2001) Group B streptococcal disease in nonpregnant adults. Clin Infect Dis 33:556–561. DOI 10.1086/322696
Lancefield RC, Freimer EH (1966) Type-specific polysaccharide antigens of group B streptococci. J Hyg (Lond) 64:191–203
Slotved HC, Kong F, Lambertsen L, Sauer S, Gilbert GL (2007) A proposed new Streptococcus agalactiae serotype, serotype IX. J Clin Microbiol 45:2929–2936. DOI 10.1128/JCM.00117-07
Baker CJ, Kasper DL (1976) Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med 294:753–756
Baker CJ, Rench MA, Paoletti LC, Edwards MS (2007) Dose-response to type V group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine in healthy adults. Vaccine 25:55–63. DOI 10.1016/j.vaccine.2006.07.018
Baker CJ, Rench MA, Fernandez M, Paoletti LC, Kasper DL, Edwards MS (2003) Safety and immunogenicity of a bivalent group B streptococcal conjugate vaccine for serotypes II and III. J Infect Dis 188:66–73. DOI 10.1086/375536
Fluegge K, Supper S, Siedler A, Berner R (2005) Serotype distribution of invasive group B streptococcal isolates in infants: results from a nationwide active laboratory surveillance study over 2 years in Germany. Clin Infect Dis 40:760–763. DOI 10.1086/427942
Harrison LH, Elliott JA, Dwyer DM, Libonati JP, Ferrieri P, Billmann L, Schuchat A (1998) Serotype distribution of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. Maryland Emerging Infections Program. J Infect Dis 177:998–1002
Lindahl G, Stalhammar-Carlemalm M, Areschoug T (2005) Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin Microbiol Rev 18:102–127. DOI 10.1128/CMR.18.1.102-127.2005
Bevanger L, Kvam AI, Maeland JA (1995) A Streptococcus agalactiae R protein analysed by polyclonal and monoclonal antibodies. APMIS 103:731–736
Smith BL, Flores A, Dechaine J, Krepela J, Bergdall A, Ferrieri P (2004) Gene encoding the group B streptococcal protein R4, its presence in clinical reference laboratory isolates & R4 protein pepsin sensitivity. Indian J Med Res 119(suppl):213–220
Stalhammar-Carlemalm M, Stenberg L, Lindahl G (1993) Protein rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J Exp Med 177:1593–1603. DOI 10.1084/jem.177.6.1593
Larsson C, Lindroth M, Nordin P, Stalhammar-Carlemalm M, Lindahl G, Krantz I (2006) Association between low concentrations of antibodies to protein alpha and Rib and invasive neonatal group B streptococcal infection. Arch Dis Child Fetal Neonatal Ed 91:F403–F408. DOI 10.1136/adc.2005.090472
Bevanger L, Maeland JA (1977) Type classification of group B streptococci by the fluorescent antibody test. Acta Pathol Microbiol Scand [B] 85B:357–362
Kong F, Gowan S, Martin D, James G, Gilbert GL (2002) Serotype identification of group B streptococci by PCR and sequencing. J Clin Microbiol 40:216–226. DOI 10.1128/JCM.40.1.216-226.2002
Borchardt SM, Foxman B, Chaffin DO, Rubens CE, Tallman PA, Manning SD, Baker CJ, Marrs CF (2004) Comparison of DNA dot blot hybridization and lancefield capillary precipitin methods for group B streptococcal capsular typing. J Clin Microbiol 42:146–150. DOI 10.1128/JCM.42.1.146-150.2004
Moyo SR, Maeland JA, Bevanger L (1999) Comparison of three different methods in monoclonal antibody-based detection of Streptococcus agalactiae protein serotype markers. APMIS 107:263–269
Zeng X, Kong F, Morgan J, Gilbert GL (2006) Evaluation of a multiplex PCR-based reverse line blot-hybridization assay for identification of serotype and surface protein antigens of Streptococcus agalactiae. J Clin Microbiol 44:3822–3825. DOI 10.1128/JCM.01232-06
Creti R, Fabretti F, Orefici G, von HC (2004) Multiplex PCR assay for direct identification of group B streptococcal alpha-protein-like protein genes. J Clin Microbiol 42:1326–1329. DOI 10.1128/JCM.42.3.1326-1329.2004
Aavitsland P, Hoiby EA, Lystad A (1996) Systemic group B streptococcal disease in neonates and young infants in Norway 1985–94. Acta Paediatr 85:104–105. DOI 10.1111/j.1651-2227.1996.tb13900.x
Puopolo KM, Madoff LC, Eichenwald EC (2005) Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics 115:1240–1246. DOI 10.1542/peds.2004-2275
Fluegge K, Siedler A, Heinrich B, Schulte-Moenting J, Moennig MJ, Bartels DB, Dammann O, von KR, Berner R (2006) Incidence and clinical presentation of invasive neonatal group B streptococcal infections in Germany. Pediatrics 117:e1139–e1145. DOI 10.1542/peds.2005-2481
Luck S, Torny M, d’Agapeyeff K, Pitt A, Heath P, Breathnach A, Russell AB (2003) Estimated early-onset group B streptococcal neonatal disease. Lancet 361:1953–1954. DOI 10.1016/S0140-6736(03)13553-2
Persson E, Berg S, Trollfors B, Larsson P, Ek E, Backhaus E, Claesson BE, Jonsson L, Radberg G, Ripa T, Johansson S (2004) Serotypes and clinical manifestations of invasive group B streptococcal infections in western Sweden 1998–2001. Clin Microbiol Infect 10:791–796. DOI 10.1111/j.1469-0691.2004.00931.x
Kalliola S, Vuopio-Varkila J, Takala AK, Eskola J (1999) Neonatal group B streptococcal disease in Finland: a ten-year nationwide study. Pediatr Infect Dis J 18:806–810. DOI 10.1097/00006454-199909000-00012
Maeland JA, Bevanger L, Lyng RV (2005) Immunological markers of the R4 protein of Streptococcus agalactiae. Clin Diagn Lab Immunol 12:1305–1310. DOI 10.1128/CDLI.12.11.1305-1310.2005
Persson E, Berg S, Bevanger L, Bergh K, Valso-Lyng R, Trollfors B (2007) Characterisation of invasive group B streptococci based on investigation of surface proteins and genes encoding surface proteins. Clin Microbiol Infect 14:66–73. DOI 10.1111/j.1469-0691.2007.01877.x
Poyart C, Tazi A, Reglier-Poupet H, Billoet A, Tavares N, Raymond J, Trieu-Cuot P (2007) Multiplex PCR assay for rapid and accurate capsular typing of group B streptococci. J Clin Microbiol 45:1985–1988. DOI 10.1128/JCM.00159-07
Acknowledgements
The authors would like to thank Randi Valsoe Lyng, Department of Microbiology, St. Olavs Hospital for characterization of invasive GBS strains. Arne Brantsaeter at The Norwegian Institute of Public Health is also gratefully acknowledged for providing data on infants with invasive GBS disease.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bergseng, H., Rygg, M., Bevanger, L. et al. Invasive group B streptococcus (GBS) disease in Norway 1996–2006. Eur J Clin Microbiol Infect Dis 27, 1193–1199 (2008). https://doi.org/10.1007/s10096-008-0565-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-008-0565-8